Abstract
Due to their key role in the pathogenesis of cancer through the regulation of apoptosis, the B-cell leukemia/lymphoma-2 (BCL-2) family proteins have been an attractive target for cancer therapy for the past decades. Throughout the years, many Bcl-2 family inhibitors have been developed, with Venetoclax being now successfully used in treating hematological malignancies. Although their effectiveness in the treatment of solid tumors is yet to be established, some preclinical evidence indicates their possible clinical application. This review aims to summarize current data from completed clinical trials that used Bcl-2 protein family inhibitors as monotherapy or in combination with other agents for the treatment of solid malignancies. We managed to include clinical trials of various phases which analyze the pharmacokinetics and pharmacodynamics of the drugs, as well as the effectiveness and adverse effects. Active and recruiting clinical trials are also briefly presented and future prospects and challenges are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is defined by unregulated cell division and growth. The main pathogenetic mechanisms leading to carcinogenesis include mutations in oncogenes or tumor-suppressor genes, epigenetic or chromosomal alterations and environmental stress, often resulting in the evasion of apoptosis [1, 2]. Apoptosis is a type of programmed cell death as a response to physiological processes, abnormal stimuli or cellular stress and, therefore, prevents a defective cell from evolving to cancer [3]. It occurs through two different pathways; the intrinsic, which is triggered by the release of apoptogenic factors in the mitochondria, and the extrinsic, which is activated upon ligation of specific death receptors at the plasma membrane [3, 4].
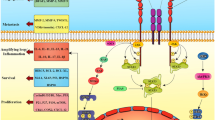
One of the main regulators of the intrinsic pathway is the B-cell leukemia/lymphoma-2 (Bcl-2), a family of regulatory proteins, which can be subclassified into different groups based on their morphology and Bcl-2 homology (BH) domain (Fig. 1). The BH3-only proteins activate BAX and BAK, which then form pores in the outer mitochondrial membrane, leading to the release of cytochrome c. This process initiates the caspase cascade pathway and eventually the apoptosis of the cell. The main action of the anti-apoptotic proteins is the inhibition of BAX, BAK and BH3-only proteins and therefore the apoptotic process [5, 6], (Fig. 2, Fig. 3).
It has been observed that in many types of cancer there is an up-regulation of anti-apoptotic proteins and a down-regulation of pro-apoptotic members of the Bcl-2 family [7]. The malignancies that were firstassociated with Bcl-2 overexpression were Chronic Lymphocytic Leukemia (CLL) and B-cell Lymphoma, hence the name Bcl-2 (B-cell leukemia/lymphoma-2 protein) [8]. Subsequently, researchers have been focusing on developing drugs that target the anti-apoptotic proteins, as an alternative approach to anticancer therapeutics. Many Bcl-2 protein family inhibitors have been developed over the past years, including venetoclax (ABT-199), navitoclax (ABT-263), obatoclax (GX15-070), oblimersen sodium (G3139), etc., and are mostly used in leukemia, lymphomas, and other hematological malignancies [9]. In particular, venetoclax was proved to be a major breakthrough in treating drug-resistant CLL, as it induces the intrinsic apoptotic cascade independently of TP53 expression [10].
Despite major breakthroughs in hematological neoplasms, the effectiveness of these drugs in solid tumors is still under investigation. Several clinical trials have been conducted regarding the role of different Bcl-2 protein family inhibitors in solid malignancies. In this narrative review we sought to provide an update on the available evidence from clinical trials of Bcl-2 inhibitors in solid malignancies and to briefly state the limitations, challenges and future goals of this anticancer approach.
Clinical investigation of bcl-2 inhibitors in solid tumor treatment
Oblimersen sodium (G3139)
Oblimersen sodium is an antisense oligonucleotide compound which targets the first 6 codons of the human Bcl-2 mRNA sequence, leading to degeneration of the Bcl-2 mRNA and decreased Bcl-2 protein production. It is the first oligonucleotide known to have an antisense effect on Bcl-2 protein translation [11]. Although it has not been approved by the Food and drug administration (FDA), many clinical trials suggest that oblimersen enhances the efficacy of cytotoxic chemotherapy especially for the treatment of CLL, multiple myeloma, malignant melanoma, and non-small cell lung cancer (NSCLC) [12]. Specifically for solid tumors, we found 16 completed clinical trials that evaluate the therapeutic potential of oblimersen in combination with other cytotoxic agents (Table 1).
In a phase I dose-escalation, multicenter study in 2000 oblimersen sodium was combined with irinotecan in patients with metastatic colorectal carcinoma to assess the pharmacokinetic behavior and the Bcl-2 protein inhibition in peripheral blood mononuclear cells (PBMCs), since preclinical studies have detected Bcl-2 overexpression in colorectal carcinoma specimens compared to normal colonic epithelium [13,14,15]. The co-administration of oblimersen and irinotecan was found to be well tolerated and moderately effective at the recommended phase II dose (RP2D). In a higher dose almost 50% of patients exhibited severe (grade 3–4) neutropenia. A decrease in Bcl-2 protein levels in PBMCs was also observed. In conclusion, these results suggest that the addition of oblimersen may increase the chemotherapeutic cytotoxicity of irinotecan, but further testing with randomized trials is needed to evaluate the real efficacy of this combination treatment [16].
It is suggested that chemoresistance in metastatic melanoma can be attributed to high antiapoptotic activity by the Bcl-2 protein family. Under these circumstances, a randomized phase III trial was conducted in 2000 to compare the effectiveness of dacarbazine with or without oblimersen in advanced malignant melanoma. The overall survival (OS) time was not improved in the arm who received combined therapy, but the progression-free survival (PFS) time was significantly increased [17, 18]. No difference in treatment outcomes was observed in patients with elevated serum lactate dehydrogenase (LDH), suggesting that LDH can be used as a biomarker for adverse prognosis. Overall, this study indicates that oblimersen sodium can enhance the efficacy of dacarbazine in patients with advanced melanoma with normal baseline LDH levels [18].
Another randomized, phase II study in 2004 assessed the antitumor activity and safety of oblimersen sodium when administered before docetaxel versus docetaxel alone among patients with castration-resistant prostate cancer (CRPC). It has been found that Bcl-2 is upregulated in prostate cancer cells, leading to androgen independence and subsequent chemoresistance to docetaxel [19, 20]. Therefore, the addition of a Bcl-2 inhibitor could possibly enhance the sensitivity of CRPC cells to docetaxel. The results though failed to show a prostate-specific antigen (PSA) response greater than 30% or a major toxic event rate less than 45% in the oblimersen-docetaxel group. The combination treatment was also associated with a higher incidence of fatigue, mucositis, and thrombocytopenia. This study highlights the possibility of improved outcomes if the target group was more specific, as observed in the study above which proved that the population with normal LDH levels exhibited better response [21].
Although some studies exhibited a synergistic effect between oblimersen and specific chemotherapeutic agents, the results were not significant enough to continue examining this drug. The fact that the last study of oblimersen sodium in solid tumors we managed to find was more than a decade ago shows that this Bcl-2 inhibitor is probably no longer used in clinical trials.
Obatoclax mesylate (GX15-070)
Unlike other more selective Bcl-2 inhibitors, obatoclax is considered a pan-Bcl-2 inhibitor, meaning that it targets all anti-apoptotic Bcl-2 family proteins [22]. Thanks to its ability to inhibit MCL-1, obatoclax was expected to have a promising antitumor effect on solid malignancies, especially in small-cell lung cancer (SCLC) where overexpression of MCL-1 is considered to cause insensitivity to other more selective Bcl-2 inhibitors [23]. In our study, we assessed five completed clinical trials which had used obatoclax mesylate in the presence of other agents for the treatment of solid cancers (Table 1).
In 2006, an open-label, phase I/II study was conducted in patients with NSCLC that relapsed after first-line platinum-based treatment. NSCLC cells are known to overexpress Bcl-2 antiapoptotic proteins [24], so the addition of a Bcl-2 inhibitor was expected to improve tumor sensitivity to taxanes. Obatoclax mesylate was combined with standard second-line NSCLC drug, docetaxel, to evaluate the tolerability and tumor response. Among the most common toxicities were neutropenia, as frequently observed with docetaxel, and transient neurologic side-effects, which is consistent with the findings of other clinical trials of obatoclax. No significant response rate was observed and thus, this study does not support further evaluation of the combination treatment in such patients [25].
In 2007, an open-label, single-arm phase II study evaluated the efficacy of combining topotecan with obatoclax mesylate in patients with relapsed SCLC. Topoisomerase inhibitors, like topotecan, trigger apoptosis by causing DNA damage to cancer cells. Antiapoptotic agents like the Bcl-2 family proteins can hinder this mechanism and lead to treatment resistance. Considering the above, a Bcl-2 inhibitor was expected to increase the chemotherapeutic effect. However, the addition of obatoclax did not manage to exceed the response rate of topotecan monotherapy in these patients. The most common adverse effects were neurologic, including ataxia and somnolence, lasting no more than 2 h. Hematologic toxicities were relatively infrequent, but more severe and required blood and platelet transfusion. Even so, it is worth mentioning that topotecan alone has a response rate as low as 7–10% in patients with platinum-refractory SCLC and, considering the small number of patients enrolled, it was not expected to observe a significantly higher efficacy [26].
In a more extended, randomized, phase II study in 2008 obatoclax mesylate was added to carboplatin/etoposide chemotherapy as first-line treatment in patients with extensive-stage SCLC. As expected, transient neurologic and psychiatric adverse effects were present in the obatoclax + carboplatin/etoposide arm, but no treatment discontinuation was needed. Unfortunately, the addition of obatoclax did not prove to significantly increase objective response rate [27].
In conclusion, obatoclax mesylate was not reported to add to the efficacy of other cytotoxic agents, neither in previously untreated nor in platinum-resistant lung cancer. To our knowledge, there are currently no recruiting clinical trials of obatoclax mesylate in patients with solid tumors.
Navitoclax (ABT-263)
Navitoclax is one of the Bcl-2 family protein inhibitors which shows a high affinity to Bcl-2, Bcl-W and Bcl-xL antiapoptotic proteins. Early preclinical studies indicate that navitoclax alone is potent to cause suppression in tumors that rely on the overexpression of antiapoptotic Bcl-2 proteins for their survival, such as SCLC, acute lymphocytic leukemia (ALL) and fibrotic diseases [28]. In this review we examined a total of 12 completed clinical trials of navitoclax alone or with the presence of other drugs in solid tumor treatment (Table 1).
In 2007, a non-randomized Phase I study was conducted to evaluate tolerability, pharmacokinetics, and efficacy of navitoclax in SCLC and other solid tumors. Overall, navitoclax was well tolerated with most of treatment-related toxicities being grade 1–2. All patients experienced thrombocytopenia, a known adverse event of drugs that inhibit Bcl-xL, which was dose-dependent and manageable. This study also indicates that in some cases of SCLC, MCL-1 rather than Bcl-2/Bcl-xL is responsible for evading apoptosis. This might explain why these tumors show insensitivity to navitoclax and other Βcl-2/Bcl-xL inhibitors. Combination of navitoclax and other drugs that downregulate the expression of MCL-1 could subsequently have promising results in these tumors [23]. Phase II included the evaluation of safety at the RP2D, as well as the preliminary efficacy of navitoclax in patients with recurrent SCLC. The results were disappointing, as limited antitumor activity was achieved and thus, the majority of the patients discontinued treatment due to disease progression. Once again, the study recommends that future studies should focus on combination therapy [29].
In a prospective, multicenter Phase II study in 2015 navitoclax was tested as a single agent on women with heavily pretreated, platinum-resistant ovarian cancer (MONAVI-GINECO study), since a promising antitumor effect on chemo-resistant ovarian cancer cells was demonstrated during preclinical trials. Thrombocytopenia was the main side-effect, but it was reversible with no significant bleeding or toxicity-related deaths. Unfortunately, navitoclax in monotherapy exhibited no significant antitumor activity. No correlation between the expression of the pro-apoptotic BIM and the anti-apoptotic MCL-1 with disease progression was observed from the analysis of tumor biopsies [30].
In the same year, another Phase Ib study evaluated the safety and feasibility of navitoclax in combination with osimertinib among patients with epidermal growth factor receptor (EGFR)-mutant NSCLC who had exhibited resistance to prior tyrosine kinase inhibitor (TKI) exposure. Preclinical studies had shown that increased apoptotic activity significantly enhances the anti-tumor effect of a third generation TKI, possibly resulting in stronger and more long-lasting tumor regression in clinical models. Early thrombocytopenia was, as expected, the major adverse effect. However, this time the combination treatment not only proved to be safe, but also demonstrated clinical efficacy. It is indicated that further investigation of Bcl-2 inhibition and osimertinib combination is needed to validate these outcomes [31].
A low platelet count was reported as the principal side-effect in almost all clinical trials of navitoclax in monotherapy or in combination treatment. This is a result of navitoclax directly inducing the apoptotic death of platelets, as it inhibits their main anti-apoptotic factor, Bcl-xL [32]. Although in such cases thrombocytopenia occurrence was transient and well-tolerated, new and improved Bcl-2 family protein inhibitors needed to be developed to limit this toxicity.
Palcitoclax (APG-1252)
Palcitoclax is a highly potent Bcl-2 family protein antagonist and, like navitoclax, targets mainly the Bcl-2 and Bcl-xL antiapoptotic proteins. It was developed in an effort to reduce on-target platelet toxicity while maintaining high anticancer activity. Preclinical studies have shown that palcitoclax can achieve tumor suppression in multiple xenograft models, including ALL, SCLC, colorectal and breast cancer [33, 34]. We examined 1 clinical trial of palcitoclax in solid tumors which was completed with available results (Table 1).
In 2017 the first in-human phase I study of palcitoclax was conducted to evaluate the safety, pharmacokinetics, and efficacy of the drug among US patients with metastatic SCLC or other solid malignancies [34]. Although palcitoclax was safe at doses lower than dose-limiting toxicity (DLT), with a relatively tolerable platelet toxicity, the supporting evidence is limited and further investigation is necessary to establish their possible antitumor effect.
AT-101 (R-(-)-gossypol acetic acid)
Gossypol, a complex compound, naturally produced by cotton plants, was first discovered in the 1950s in China, as cooking with crude cottonseed was found to cause infertility in men [35, 36]. The gossypol (-)-enantiomer, known as gossypol acetic acid or AT-101, is the more biologically active form and it effectively induces the mitochondrial apoptotic pathway by downregulating the anti-apoptotic Bcl-2 proteins, including Bcl-2, Bcl-xL, Mcl-1, and Bcl-w [37, 38]. Except for the Bcl-2 inhibition pathway, AT-101 seems to play a significant role in the regulation of other cell signaling pathways by inhibiting vascular endothelial growth factor (VEGF)-mediated angiogenesis and Apurinic/apyrimidinic endodeoxyribonuclease 1 (APE1) [39, 40]. In our study, we summarized the outcomes of 14 completed clinical trials in which AT-101 was used to treat patients with solid cancers [Table 1].
In 2007 a double- blind, multicenter, randomized phase II study was conducted to evaluate the efficacy and tolerability of AT-101 in combination with docetaxel for the treatment of relapsed NSCLC. In preclinical prostate and lung cancer models, docetaxel and AT-101 were found to have a synergistic or even additive effect. However, in this clinical trial the combination treatment did not achieve an improved PFS. The adverse effects in the docetaxel + AT-101 arm were generally the same as in the docetaxel + placebo arm, with the exception of headache, which was significantly more frequent in the docetaxel + AT-101 group (11.3% compared to 0%). An increase in median OS by 1.9 months was reported in the AT-101 group, but it did not meet statistical significance [41].
Another randomized, double-blind, placebo-controlled, phase II study also in 2007 compared the potency of AT-101 in combination with docetaxel and prednisone (ADP arm) versus docetaxel and prednisone plus placebo (placebo-DP arm) in patients with chemotherapy-naïve metastatic hormone refractory prostate cancer. The ADP arm was associated with an increased incidence in cardiac and hematologic adverse events, as well as pulmonary embolism and peripheral neuropathy. Efficacy endpoints (especially OS and ≥ 50% PSA decline) were found to be increased in the subgroup of high-risk patients of the ADP arm, suggesting that Bcl-2 family protein expression may play a more significant role in such patients. However, no statistically significant difference in OS or PFS was observed between the two arms [42].
In 2012 a new therapeutic approach to advanced laryngeal cancer was tested with the combination of platinum, docetaxel and AT-101 in a randomized, phase II study. While adverse effects were overall more manageable, AT-101 did not improve disease response [43].
In a recent study with both preclinical and clinical aspects among patients with gastroesophageal cancer the combination of AT-101 with docetaxel, fluorouracil, and radiation exhibited surprisingly encouraging results, suggesting that the proapoptotic effect of AT-101 successfully overcomes the antiapoptotic pathways of gastroesophageal cancer stem cells [44]. However, more randomized, placebo-controlled clinical trials are needed to further investigate and confirm these results.
In conclusion, AT-101 was well-tolerated in all combination treatments, but in most studies improvement in primary endpoints did not reach statistical significance.
Venetoclax (ABT-199)
In an effort to limit the adverse hematological toxicities of navitoclax, a new highly selective Bcl-2 inhibitor was introduced. Unlike other Bcl-2 inhibitors, which target more than one anti-apoptotic proteins, venetoclax is a selective inhibitor of the Bcl-2 protein and was first FDA approved in 2016 for the treatment of CLL, especially with 17p deletion [45]. It is now considered a novel drug for not only CLL, but also acute myeloid leukemia (AML) and small lymphocytic lymphoma, and it has exhibited promising results in other blood cancers, such as multiple myeloma [46]. However, the therapeutic potential of venetoclax in solid tumors is still under clinical evaluation. We assessed 2 completed clinical trials examining the use of venetoclax in different types of solid cancers with the one having available results (Table 1).
In 2018 a randomized, phase II study (VERONICA) compared the efficacy of venetoclax in combination with fulvestrant compared with fulvestrant alone in women with estrogen receptor (ER)-positive, HER2-negative, locally advanced or Metastatic Breast Cancer (MBC) who experienced disease recurrence or progression during or after treatment with CDK4/6i therapy. Preclinical studies support that Bcl-2 is overexpressed in approximately 85% of primary ER-positive breast cancer cases, so the inhibition of the antiapoptotic protein could enhance the therapeutic effect of endocrine therapy with fulvestrant in such patients. However, the clinical benefit rate and the PFS were not significantly improved in the venetoclax + fulvestrant arm. More serious (grade 3–4) adverse events were also observed in the combination treatment [47]. As of October 2020, participants in the venetoclax + fulvestrant arm have all discontinued venetoclax treatment and have continued on fulvestrant treatment alone [48].
LP-118
LP-118 is an oral selective Bcl-2/Bcl-xL inhibitor and it is the newest of all drugs we have included in this study. Its special feature is that its anti Bcl-xL activity is adjusted to the minimum so that the risk of thrombocytopenia is limited [49]. We report only one recruiting clinical trial of LP-118 as a single agent in patients with lymphoma or solid tumors and the results are expected in the forthcoming years (Table 2).
Interpretation and discussion
As noted earlier, Bcl-2 inhibitors have proven their effectiveness in treating hematological malignancies through multiple preclinical and clinical studies for many years now. Their crucial role in evading apoptosis still renders them as one of the most promising targets for cancer treatment. Early data from preclinical studies have supported that some Bcl-2 family proteins are overexpressed in many solid tumors too, raising a big question; are Bcl-2 inhibitors as effective in solid tumors as in hematological? To answer this question, we examined a great number of clinical trials using the Bcl-2 inhibitors oblimersen sodium, obatoclax mesylate, navitoclax, palcitoclax, AT-101, venetoclax and LP-118. The most frequently utilized approach seems to be the co-administration of a Bcl-2 inhibitor with other anticancer agents, such as but not limited to conventional chemotherapy.
Oblimersen sodium and obatoclax mesylate did not manage to demonstrate statistically significant clinical efficacy in any case of solid malignancies. Further investigation is not suggested, and we report no recruiting clinical trials of these agents. AT-101 was found to successfully target cancer stem cells of gastroesophageal cancer in one clinical trial [44] but failed to prove significant antitumor effect in all other trials. Navitoclax was mainly tested in lung cancer and was expected to sufficiently increase apoptotic activity and chemosensitivity, as demonstrated in preclinical studies. Unfortunately, so far only one study has managed to prove statistically significant antitumor response [31]. Regarding palcitoclax, we cannot reach any conclusions based on one completed trial. Nevertheless, the first results as a monotherapy in metastatic SCLC were promising and further investigation is warranted [34]. Venetoclax is a newly developed drug and, therefore, little research is still conducted concerning solid malignancies. Many clinical trials are currently evaluating the efficacy of adding venetoclax to other agents in many types of solid tumors, such as CRPC, breast cancer and neuroblastoma.
In terms of overall safety and toxicity, the adverse events that occurred could be explained by both the co-administered agent and the Bcl-2 inhibitor itself. More specifically, common toxicities included severe neutropenia and thrombocytopenia (grade 3 and 4), anemia, lymphopenia, fatigue, diarrhea, vomiting, hepatic and metabolic disturbances. However, few instances of serious adverse events such as myocardial infarction and pulmonary embolism were recorded. Neurologic adverse events were reported only in groups receiving palcitoclax, but were transient and did not require any intervention. Thrombocytopenia was more prominent with navitoclax and was attributed to direct inhibition of Bcl-xL. Results from most randomized trials exhibited a higher incidence of hematologic toxicities among patients receiving combination treatment compared to conventional chemotherapy. Although these adverse events were manageable, it is suggested that the addition of Bcl-2 inhibitors to chemotherapeutic agents can aggravate the hematologic toxicities.
The main hindrance in evaluating the efficacy of these drugs was the limited number of participants, which predisposes to statistical errors, while a non-negligible number of patients had other comorbidities that could potentially aggravate the rate and severity of occurring adverse events. It is also worth mentioning that most trials were completed 10 or more years ago. Since then, patient care has evolved substantially and a variety of new chemotherapeutic agents has been released. These observations emphasize the necessity for renewed and more extended randomized clinical trials with stricter eligibility criteria regarding patients’ health status, as a means of objectively identifying clinical efficacy and safety issues.
Conclusion
To our knowledge, this is the most recent review to summarize clinical trials of Bcl-2 inhibitors on solid tumors. It is evident that Bcl-2 inhibitors do not seem to be as efficient against solid tumors as they are against hematological cancers, when used as single agents. However, in combination with other anticancer drugs they are likely to enhance their antitumor effect while maintaining a good safety profile. We also highlight the importance of further preclinical research which may pave the way for new, more potent combinations of Bcl-2 inhibitors with other targeted agents. Currently 10 clinical trials are recruiting with their primary endpoints being the assessment of maximum tolerated dose, safety and adverse events, pharmacokinetic, pharmacodynamic parameters and effectiveness (Table 2). Their results are anticipated in the forthcoming years and should add useful information in our arsenal against cancer.
Data availability
Data are included in the manuscript.
References
Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. https://doi.org/10.1038/nm1087.
Sarkar S, Horn G, Moulton K, Oza A, Byler S, Kokolus S, et al. Cancer development, progression, and therapy: an epigenetic overview. Int J Mol Sci. 2013;14:21087–113. https://doi.org/10.3390/ijms141021087.
Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. https://doi.org/10.1038/35037734.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. https://doi.org/10.1038/35037710.
Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25:56–64. https://doi.org/10.1038/cdd.2017.183.
Ryan CE, Davids MS. BCL-2 inhibitors, present and future. Cancer J. 2019;25:401–9. https://doi.org/10.1097/ppo.0000000000000408.
Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. https://doi.org/10.1038/348334a0.
Pentimalli F. BCL2: a 30-year tale of life, death and much more to come. Cell Death Differ. 2018;25:7–9. https://doi.org/10.1038/cdd.2017.189.
Hafezi S, Rahmani M. Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers (Basel). 2021;13:1292. https://doi.org/10.3390/cancers13061292.
Anderson MA, Deng J, Seymour JF, Tam C, Kim SY, Fein J, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127:3215–24. https://doi.org/10.1182/blood-2016-01-688796.
Frankel SR. Oblimersen sodium (G3139 Bcl-2 antisense oligonucleotide) therapy in Waldenstrom’s macroglobulinemia: a targeted approach to enhance apoptosis. Semin Oncol. 2003;30:300–4. https://doi.org/10.1053/sonc.2003.50041.
Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. https://doi.org/10.1089/108729002760220798.
Nakamura T, Nomura S, Sakai T, Nariya S. Expression of bcl-2 oncoprotein in gastrointestinal and uterine carcinomas and their premalignant lesions. Hum Pathol. 1997;28:309–15. https://doi.org/10.1016/s0046-8177(97)90129-5.
Valassiadou KE, Stefanaki K, Tzardi M, Datseris G, Georgoulias V, Melissas J, et al. Immunohistochemical expression of p53, bcl-2, mdm2 and waf1/p21 proteins in colorectal adenocarcinomas. Anticancer Res. 1997;17:2571–6.
Mueller J, Mueller E, Hoepner I, Jütting J, Bethke B, Stolte M, et al. Expression of bcl-2 and p53 in de novo and ex-adenoma colon carcinoma: a comparative immunohistochemical study. J Pathol. 1996;180:259–65. https://doi.org/10.1002/(sici)1096-9896(199611)180:3%3C259::aid-path654%3E3.0.co;2-1.
Mita MM, Ochoa L, Rowinsky EK, Kuhn J, Schwartz G, Hammond LA, et al. A phase I, pharmacokinetic and biologic correlative study of oblimersen sodium (Genasense™, G3139) and irinotecan in patients with metastatic colorectal cancer. Ann Oncol. 2006;17:313–21. https://doi.org/10.1093/annonc/mdj067.
Oblimersen. Drugs in R & D. 2007;8:321–34. https://doi.org/10.2165/00126839-200708050-00006
Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the oblimersen melanoma study group. J Clin Oncol. 2006;24:4738–45. https://doi.org/10.1200/jco.2006.06.0483.
Gleave ME, Miayake H, Goldie J, Nelson C, Tolcher A. Targeting bcl-2 gene to delay androgen-independent progression and enhance chemosensitivity in prostate cancer using antisense bcl-2 oligodeoxynucleotides. Urology. 1999;54:36–46. https://doi.org/10.1016/s0090-4295(99)00453-7.
Goodin S, Rao Kv, DiPaola RS. State-of-the-art treatment of metastatic hormone-refractory prostate cancer. Oncologist. 2002;7:360–70. https://doi.org/10.1634/theoncologist.7-4-360.
Sternberg CN, Dumez H, van Poppel H, Skoneczna I, Sella A, Daugaard G, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:1264–9. https://doi.org/10.1093/annonc/mdn784.
Vogler M. Targeting BCL2-proteins for the treatment of solid tumours. Adv Med. 2014;2014:1–14. https://doi.org/10.1155/2014/943648.
Gandhi L, Camidge DR, de Oliveira MR, Bonomi P, Gandara D, Khaira D, et al. Phase I Study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. https://doi.org/10.1200/jco.2010.31.6208.
Berrieman HK, Smith L, O’Kane SL, Campbell A, Lind MJ, Cawkwell L. The expression of Bcl-2 family proteins differs between nonsmall cell lung carcinoma subtypes. Cancer. 2005;103:1415–9. https://doi.org/10.1002/cncr.20907.
Chiappori A, Williams C, Northfelt DW, Adams JW, Malik S, Edelman MJ, et al. Obatoclax mesylate, a Pan–Bcl-2 inhibitor, in combination with docetaxel in a phase 1/2 trial in relapsed non–small-cell lung cancer. J Thorac Oncol. 2014;9:121–5. https://doi.org/10.1097/jto.0000000000000027.
Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74:481–5. https://doi.org/10.1016/j.lungcan.2011.05.005.
Langer CJ, Albert I, Ross HJ, Kovacs P, Blakely LJ, Pajkos G, et al. Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer. Lung Cancer. 2014;85:420–8. https://doi.org/10.1016/j.lungcan.2014.05.003.
Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: navitoclax as a pro-apoptotic and anti-fibrotic agent. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.564108.
Rudin CM, Hann CL, Garon EB, de Oliveira MR, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. https://doi.org/10.1158/1078-0432.ccr-11-3090.
Joly F, Fabbro M, Follana P, Lequesne J, Medioni J, Lesoin A, et al. A phase II study of navitoclax (ABT-263) as single agent in women heavily pretreated for recurrent epithelial ovarian cancer: the MONAVI—GINECO study. Gynecol Oncol. 2022;165:30–9. https://doi.org/10.1016/j.ygyno.2022.01.021.
Bertino EM, Gentzler RD, Clifford S, Kolesar J, Muzikansky A, Haura EB, et al. Phase IB study of osimertinib in combination with navitoclax in EGFR -mutant NSCLC following resistance to initial EGFR therapy (ETCTN 9903). Clin Cancer Res. 2021;27:1604–11. https://doi.org/10.1158/1078-0432.ccr-20-4084.
Debrincat MA, Pleines I, Lebois M, Lane RM, Holmes ML, Corbin J, et al. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis. 2015;6:e1721–e1721. https://doi.org/10.1038/cddis.2015.97.
Yao W, Bai L, Wang S, Zhai Y, Sun S-Y. Mcl-1 levels critically impact the sensitivities of human colorectal cancer cells to APG-1252-M1, a novel Bcl-2/Bcl-XL dual inhibitor that induces Bax-dependent apoptosis. Neoplasia. 2022;29:100798. https://doi.org/10.1016/j.neo.2022.100798.
Lakhani NJ, Rasco DW, Zeng Q, Tang Y, Liang Z, Wang H, et al. First-in-human study of palcitoclax (APG-1252), a novel dual Bcl-2/Bcl-xL inhibitor, demonstrated advantages in platelet safety while maintaining anticancer effect in US patients with metastatic solid tumors. J Clin Oncol. 2020;38:3509–3509.
Renner O, Mayer M, Leischner C, Burkard M, Berger A, Lauer UM, et al. Systematic review of gossypol/AT-101 in cancer clinical trials. Pharmaceuticals. 2022;15:144. https://doi.org/10.3390/ph15020144.
Hoshiai H, Uehara S, Mori R, Nagaike F, Tsuiki A, Suzuki M. Gossypol as oral contraceptive for male: trial case report. Tohoku J Exp Med. 1982;138:275–80. https://doi.org/10.1620/tjem.138.275.
Cao H, Sethumadhavan K, Cao F, Wang TTY. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci Rep. 2021;11:5922. https://doi.org/10.1038/s41598-021-84970-8.
Zeng Y, Ma J, Xu L, Wu D. Natural product gossypol and its derivatives in precision cancer medicine. Curr Med Chem. 2019;26:1849–73. https://doi.org/10.2174/0929867324666170523123655.
Pang X, Wu Y, Wu Y, Lu B, Chen J, Wang J, et al. (−)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol Cancer Ther. 2011;10:795–805. https://doi.org/10.1158/1535-7163.mct-10-0936.
Wang D, Li M, Sui J, Ren T, Li Z, Zhang L, et al. Identification of a novel potential antitumor activity of gossypol as an APE1/Ref-1 inhibitor. Drug Des Devel Ther. 2014. https://doi.org/10.2147/dddt.s62963.
Ready N, Karaseva NA, Orlov Sv, Luft Av, Popovych O, Holmlund JT, et al. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J Thorac Oncol. 2011;6:781–5. https://doi.org/10.1097/jto.0b013e31820a0ea6.
Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:1803–8. https://doi.org/10.1093/annonc/mdr555.
Swiecicki P, Bellile E, Casper K, Malloy KM, Kupfer R, Spector ME, et al. A randomized trial of laryngeal organ preservation evaluating two cycles of induction chemotherapy with platinum, docetaxel, and a novel Bcl-xL inhibitor. J Clin Oncol. 2019;37:6066–6066.
Song S, Chen Q, Li Y, Lei G, Scott A, Huo L, et al. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut. 2021;70:2238–48. https://doi.org/10.1136/gutjnl-2020-321175.
Deeks ED. Venetoclax: first global approval. Drugs. 2016;76:979–87. https://doi.org/10.1007/s40265-016-0596-x.
Juárez-Salcedo LM, Desai V, Dalia S. Venetoclax: evidence to date and clinical potential. Drugs Context. 2019;8:1–13. https://doi.org/10.7573/dic.212574.
Lindeman GJ, Bowen R, Jerzak KJ, Song X, Decker T, Boyle FM, et al. Results from VERONICA: a randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC). J Clin Oncol. 2021;39:1004–1004.
Lindeman GJ, Fernando TM, Bowen R, Jerzak KJ, Song X, Decker T, et al. VERONICA: randomized phase II study of fulvestrant and venetoclax in ER-Positive metastatic breast cancer post-CDK4/6 inhibitors—efficacy, safety, and biomarker results. Clin Cancer Res. 2022;28:3256–67. https://doi.org/10.1158/1078-0432.ccr-21-3811.
Ravikrishnan J, Muhowski EM, Lai T-H, Misra S, Diaz Rohena D, Tan F, et al. Characterization of LP-118, a novel small molecule inhibitor of Bcl-2 and Bcl-Xl in chronic lymphocytic leukemia resistant to venetoclax. Blood. 2021;138:679–679. https://doi.org/10.1182/blood-2021-151852.
Funding
Open access funding provided by HEAL-Link Greece. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare that they have no financial or non-financial interests.
Author information
Authors and Affiliations
Contributions
Conceptualization: EK, IT; Literature search and data analysis: KD, KK, IK; Writing—original draft: I-AK, IP, ET Writing—review and editing: IP, ET, EK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ploumaki, I., Triantafyllou, E., Koumprentziotis, IA. et al. Bcl-2 pathway inhibition in solid tumors: a review of clinical trials. Clin Transl Oncol 25, 1554–1578 (2023). https://doi.org/10.1007/s12094-022-03070-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03070-9