Abstract
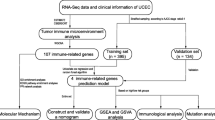
Radiotherapy is the main treatment for cervical cancer. It is usually applied alone or in combination with surgery and/or chemotherapy. To explore the association between immune microenvironment of cervical cancer and radiotherapy response, we collected 20 paired cervical cancer tumor samples before and after radiotherapy and partial clinical information. With paired-end RNA-seq, we quantified the immune infiltration and tumor purity of these samples, and obtained 6350 differentially expressed genes before and after radiotherapy. With the help of R language, the function enrichment analysis and 22 immune cells infiltration analysis were carried out. Moreover, we built a random forest model based on the immune microenvironment to predict the short-term efficacy of radiotherapy. We found that the effect of radiotherapy on the immune microenvironment of stage III and IV cervical cancer patients was weaker than that of stage I and II cervical cancer patients. Radiotherapy can significantly reduce the tumor purity and increase immune infiltration. The proportions of the immune infiltrating cells are predictive of the radiotherapy efficacy. In addition, the local mucositis caused by radiotherapy can improve the curative effect of radiotherapy.









Similar content being viewed by others
Data availability
Not applicable.
References
McGuire S. World cancer report 2014. Geneva, Switzerland: world health organization, international agency for research on cancer, WHO press, 2015. Adv Nutr. 2016;7:418–9. https://doi.org/10.3945/an.116.012211.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:64–84. https://doi.org/10.6004/jnccn.2019.0001.
Kim YJ, Lee KJ, Park KR, Kim J, Jung W, Lee R, et al. Prognostic analysis of uterine cervical cancer treated with postoperative radiotherapy: importance of positive or close parametrial resection margin. Radiat Oncol J. 2015;33:109–16. https://doi.org/10.3857/roj.2015.33.2.109.
Couvreur K, Naert E, De Jaeghere E, Tummers P, Makar A, De Visschere P, et al. Neo-adjuvant treatment of adenocarcinoma and squamous cell carcinoma of the cervix results in significantly different pathological complete response rates. BMC Cancer. 2018;18:1101. https://doi.org/10.1186/s12885-018-5007-0.
Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214:22–30. https://doi.org/10.1016/j.ajog.2015.07.022.
Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12:526–42. https://doi.org/10.1038/nrd4003.
Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–37. https://doi.org/10.1002/cncr.21324.
Brown JM. Therapeutic targets in radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:319–26. https://doi.org/10.1016/s0360-3016(00)01482-6.
Tofilon PJ, Saxman S, Coleman CN. Molecular targets for radiation therapy: bringing preclinical data into clinical trials. Clin Cancer Res. 2003;9:3518–20.
Loi M, Desideri I, Greto D, Mangoni M, Sottili M, Meattini I, et al. Radiotherapy in the age of cancer immunology: current concepts and future developments. Crit Rev Oncol Hematol. 2017;112:1–10. https://doi.org/10.1016/j.critrevonc.2017.02.002.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. https://doi.org/10.1056/NEJMoa022152.
Manda K, Glasow A, Paape D, Hildebrandt G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol. 2012;2:102. https://doi.org/10.3389/fonc.2012.00102.
Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. https://doi.org/10.1146/annurev.immunol.21.120601.141107.
Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. https://doi.org/10.1111/j.0105-2896.2010.00899.x.
Hugues S. Dynamics of dendritic cell-T cell interactions: a role in T cell outcome. Semin Immunopathol. 2010;32:227–38. https://doi.org/10.1007/s00281-010-0211-2.
Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Can Res. 1996;56:5150–5.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59. https://doi.org/10.1038/s41568-021-00347-z.
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–12. https://doi.org/10.1002/jcp.24260.
Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front Immunol. 2014;5:127. https://doi.org/10.3389/fimmu.2014.00127.
Chen Y, Li ZY, Zhou GQ, Sun Y. An immune-related gene prognostic index for head and neck squamous cell carcinoma. Clin Cancer Res. 2021;27:330–41. https://doi.org/10.1158/1078-0432.ccr-20-2166.
Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as a “game changer” in cancer radiotherapy. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20133212.
Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2022;12:108–33. https://doi.org/10.1158/2159-8290.cd-21-0003.
Yang X, Ren H, Fu J. Combinations of radiotherapy with immunotherapy in cervical cancer. J Cancer. 2022;13:1480–9. https://doi.org/10.7150/jca.65074.
Van Meir H, Nout RA, Welters MJ, Loof NM, de Kam ML, van Ham JJ, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology. 2017;6: e1267095. https://doi.org/10.1080/2162402x.2016.1267095.
Zou R, Gu R, Yu X, Hu Y, Yu J, Xue X, et al. Characteristics of infiltrating immune cells and a predictive immune model for cervical cancer. J Cancer. 2021;12:3501–14. https://doi.org/10.7150/jca.55970.
Giraldo NA, Becht E, Vano Y, Petitprez F, Lacroix L, Validire P, et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017;23:4416–28. https://doi.org/10.1158/1078-0432.ccr-16-2848.
Scott DW, Chan FC, Hong F, Rogic S, Tan KL, Meissner B, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700. https://doi.org/10.1200/jco.2012.43.4589.
Muris JJ, Meijer CJ, Cillessen SA, Vos W, Kummer JA, Bladergroen BA, et al. Prognostic significance of activated cytotoxic T-lymphocytes in primary nodal diffuse large B-cell lymphomas. Leukemia. 2004;18:589–96. https://doi.org/10.1038/sj.leu.2403240.
Fortis SP, Sofopoulos M, Sotiriadou NN, Haritos C, Vaxevanis CK, Anastasopoulou EA, et al. Differential intratumoral distributions of CD8 and CD163 immune cells as prognostic biomarkers in breast cancer. J Immunother Cancer. 2017;5:39. https://doi.org/10.1186/s40425-017-0240-7.
Petitprez F, Fossati N, Vano Y, Freschi M, Becht E, Lucianò R, et al. PD-L1 expression and CD8(+) T-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur Urol Focus. 2019;5:192–6. https://doi.org/10.1016/j.euf.2017.05.013.
Droeser R, Zlobec I, Kilic E, Güth U, Heberer M, Spagnoli G, et al. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134. https://doi.org/10.1186/1471-2407-12-134.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. https://doi.org/10.1016/j.immuni.2013.06.014.
Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–84. https://doi.org/10.1126/science.1224922.
Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–87. https://doi.org/10.1016/j.semcancer.2007.06.009.
Zhang Y, Yu M, Jing Y, Cheng J, Zhang C, Cheng L, et al. Baseline immunity and impact of chemotherapy on immune microenvironment in cervical cancer. Br J Cancer. 2021;124:414–24. https://doi.org/10.1038/s41416-020-01123-w.
Carlson DJ, Stewart RD, Li XA, Jennings K, Wang JZ, Guerrero M. Comparison of in vitro and in vivo alpha/beta ratios for prostate cancer. Phys Med Biol. 2004;49:4477–91. https://doi.org/10.1088/0031-9155/49/19/003.
Gong Z, Zhang J, Guo W. Tumor purity as a prognosis and immunotherapy relevant feature in gastric cancer. Cancer Med. 2020;9:9052–63. https://doi.org/10.1002/cam4.3505.
Mao Y, Feng Q, Zheng P, Yang L, Liu T, Xu Y, et al. Low tumor purity is associated with poor prognosis, heavy mutation burden, and intense immune phenotype in colon cancer. Cancer Manag Res. 2018;10:3569–77. https://doi.org/10.2147/cmar.s171855.
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921-r925. https://doi.org/10.1016/j.cub.2020.06.081.
Deng Y, Song Z, Huang L, Guo Z, Tong B, Sun M, et al. Tumor purity as a prognosis and immunotherapy relevant feature in cervical cancer. Aging. 2021;13:24768–85. https://doi.org/10.18632/aging.203714.
Tao Z, Gao J, Qian L, Huang Y, Zhou Y, Yang L, et al. Factors associated with acute oral mucosal reaction induced by radiotherapy in head and neck squamous cell carcinoma: a retrospective single-center experience. Medicine. 2017;96: e8446. https://doi.org/10.1097/md.0000000000008446.
Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol. 2015;51:875–9. https://doi.org/10.1016/j.oraloncology.2015.06.008.
Epstein JB, Schubert MM. Oral mucositis in myelosuppressive cancer therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:273–6. https://doi.org/10.1016/s1079-2104(99)70026-0.
Harris DJ. Cancer treatment-induced mucositis pain: strategies for assessment and management. Ther Clin Risk Manag. 2006;2:251–8. https://doi.org/10.2147/tcrm.2006.2.3.251.
Funding
This work was supported by National Natural Science Foundation of China (82073339; 62072058), Project of Wuxi Health Committee (Q202015) and Scientific Projects of Changzhou Medical Center raised by Nanjing Medical University (CMCB202201).
Author information
Authors and Affiliations
Contributions
ZS, XL, HL, JL conceived and designed the experiment, and analyzed the data. JS, SZ collected clinical cervical cancer samples. ZS, XL wrote this paper. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Standardize treatment according to NCCN guidelines. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in the case report.
Conflict of interest
The author is not required to declare a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z., Liu, X., Song, J. et al. Immune infiltration could predict the efficacy of short-term radiotherapy in patients with cervical cancer. Clin Transl Oncol 25, 1353–1367 (2023). https://doi.org/10.1007/s12094-022-03033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03033-0