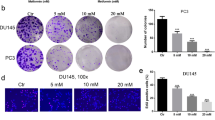
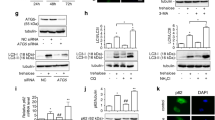
Abstract
Prostate cancer (PCa) is the second leading cause of cancer deaths in men. Unfortunately, a very limited number of drugs are available for the relapsed and advanced stages of PCa, adding only a few months to survival; therefore, it is vital to develop new drugs. 5´ AMP-activated protein kinase (AMPK) is a master regulator of cell metabolism. It plays a significant role in the metabolism of PCa; hence, it can serve well as a treatment option for the advanced stages of PCa. However, whether this pathway contributes to cancer cell survival or death remains unknown. The present study reviews the possible pathways by which AMPK plays role in the advanced stages of PCa, drug resistance, and metastasis: (1) AMPK has a contradictory role in promoting glycolysis and the Warburg effect which are correlated with cancer stem cells (CSCs) survival and advanced PCa. It exerts its effect by interacting with hypoxia-induced factor 1 (HIF1) α, pyruvate kinase 2 (PKM2), glucose transporter (GLUT) 1 and pyruvate dehydrogenase complex (PDHC), which are key regulators of glycolysis; however, whether it promotes or discourage glycolysis is not conclusive. It can also exert an anti-CSC effect by negative regulation of NANOG and epithelial–mesenchymal transition (EMT) transcription factors, which are the major drivers of CSC maintenance; (2) the regulatory effect of AMPK on autophagy is also noticeable. Androgen receptors’ expression increases AMPK activation through Calcium/calmodulin-dependent protein kinase 2 (CaMKK2) and induces autophagy. In addition, AMPK itself increases autophagy by downregulating the mammalian target of rapamycin complex (mTORC). However, whether increased autophagy inhibits or promotes cell death and drug resistance is contradictory. This study reveals that there are numerous pathways other than cell metabolism by which AMPK exerts its effects in the advanced stages of PCa, making it a priceless treatment target. Finally, we mention some drugs developed to treat the advanced stages of PCa by acting on AMPK.


Similar content being viewed by others
Code availability
Not applicable.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Zhuo L, Cheng Y, Pan Y, Zong J, Sun W, Xu L, et al. Prostate cancer with bone metastasis in Beijing: an observational study of prevalence, hospital visits and treatment costs using data from an administrative claims database. BMJ Open. 2019;9(6): e028214.
Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res. 2018;7:1513.
Patil N, Gaitonde K. Clinical perspective of prostate cancer. top Magn Reson Imaging. 2016;25(3):103–8.
Tennakoon JB, Shi Y, Han JJ, Tsouko E, White MA, Burns AR, et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch. Oncogene. 2014;33(45):5251–61.
Zadra G, Priolo C, Patnaik A, Loda M. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin Cancer Res. 2010;16(13):3322–8.
Sánchez BG, Bort A, Vara-Ciruelos D, Díaz-Laviada I. Androgen deprivation induces reprogramming of prostate cancer cells to stem-like cells. Cells. 2020;9(6):1441.
Mamouni K, Kallifatidis G, Lokeshwar BL. Targeting mitochondrial metabolism in prostate cancer with triterpenoids. Int J Mol Sci. 2021;22(5):2466.
Cai Z, Deng Y, Ye J, Zhuo Y, Liu Z, Liang Y, et al. Aberrant expression of citrate synthase is linked to disease progression and clinical outcome in prostate cancer. Cancer Manag Res. 2020;12:6149–63.
Wang Z, Wang N, Liu P, Xie X. AMPK and cancer. Exp Suppl. 2016;107:203–26.
Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat Rev Urol. 2017;14(3):164–80.
Chhipa RR, Wu Y, Mohler JL, Ip C. Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress. Cell Signal. 2010;22(10):1554–61.
Zhao Y, Xingbin H, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 2017;16:79.
Mitani T, Minami M, Harada N, Ashida H, Yamaji R. Autophagic degradation of the androgen receptor mediated by increased phosphorylation of p62 suppresses apoptosis in hypoxia. Cell Signal. 2015;27(10):1994–2001.
Saini N, Yang X. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai). 2018;50(2):133–43.
Yang YC, Chien MH, Liu HY, Chang YC, Chen CK, Lee WJ, et al. Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett. 2018;421:28–40.
Robles-Flores M, Moreno-Londoño AP, Castañeda-Patlán MC. Signaling pathways involved in nutrient sensing control in cancer stem cells: an overview. Front Endocrinol. 2021;12(219):627745.
Takayama K-I, Kosaka T, Suzuki T, Hongo H, Oya M, Fujimura T, et al. Subtype-specific collaborative transcription factor networks are promoted by OCT4 in the progression of prostate cancer. Nat Commun. 2021;12(1):3766.
Guo W, Zhang Z, Li G, Lai X, Gu R, Xu W, et al. Pyruvate kinase M2 promotes prostate cancer metastasis through regulating ERK1/2-COX-2 signaling. Front Oncol. 2020;10: 544288.
Zhu Z, Tang G, Yan J. MicroRNA-122 regulates docetaxel resistance of prostate cancer cells by regulating PKM2. Exp Ther Med. 2020;20(6):247.
Hasan D, Gamen E, Abu Tarboush N, Ismail Y, Pak O, Azab B. PKM2 and HIF-1α regulation in prostate cancer cell lines. PLoS ONE. 2018;13(9): e0203745.
Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54.
Zhang Q, Han Z, Zhu Y, Chen J, Li W. Role of hypoxia inducible factor-1 in cancer stem cells (Review). Mol Med Rep. 2021;23(1):1.
Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29(4):713–21.
Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4(10): e875.
Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, García-Echeverría C, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106(1):268–73.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51.
Yoshida GJ, Saya H. EpCAM expression in the prostate cancer makes the difference in the response to growth factors. Biochem Biophys Res Commun. 2014;443(1):239–45.
Skvortsov S, Skvortsova I-I, Tang DG, Dubrovska A. Concise review: prostate cancer stem cells: current understanding. Stem Cells. 2018;36(10):1457–74.
Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17(4):319–32.
Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells. 2020;9(1):217.
Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A. 2013;110(3):972–7.
Zhang HH, Guo XL. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol. 2016;78(1):13–26.
Wang X, Jin J, Wan F, Zhao L, Chu H, Chen C, et al. AMPK promotes SPOP-mediated NANOG degradation to regulate prostate cancer cell stemness. Dev Cell. 2019;48(3):345-60.e7.
Hart PC, Mao M, de Abreu ALP, Ansenberger-Fricano K, Ekoue DN, Ganini D, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6:6053.
Lee YG, Nam Y, Shin KJ, Yoon S, Park WS, Joung JY, et al. Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer. Cancer Lett. 2020;471:72–87.
Han F, Li C-F, Cai Z, Zhang X, Jin G, Zhang W-N, et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat Commun. 2018;9(1):4728.
Wu CA, Chao Y, Shiah SG, Lin WW. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim Biophys Acta. 2013;1833(5):1147–56.
Townsend LK, Weber AJ, Barbeau PA, Holloway GP, Wright DC. Reactive oxygen species-dependent regulation of pyruvate dehydrogenase kinase-4 in white adipose tissue. Am J Physiol Cell Physiol. 2020;318(1):C137–49.
Lin C, Salzillo TC, Bader DA, Wilkenfeld SR, Awad D, Pulliam TL, et al. Prostate cancer energetics and biosynthesis. Adv Exp Med Biol. 2019;1210:185–237.
Zhong Y, Li X, Ji Y, Li X, Li Y, Yu D, et al. Pyruvate dehydrogenase expression is negatively associated with cell stemness and worse clinical outcome in prostate cancers. Oncotarget. 2017;8(8):13344–56.
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–24.
Moldogazieva NT, Mokhosoev IM, Terentiev AA. Metabolic heterogeneity of cancer cells: an interplay between HIF-1, GLUTs, and AMPK. Cancers (Basel). 2020;12(4):862.
Park HU, Suy S, Danner M, Dailey V, Zhang Y, Li H, et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8(4):733–41.
Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN Jr, et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33(36):4521–30.
Lin C, Blessing AM, Pulliam TL, Shi Y, Wilkenfeld SR, Han JJ, et al. Inhibition of CAMKK2 impairs autophagy and castration-resistant prostate cancer via suppression of AMPK-ULK1 signaling. bioRxiv. 2020;40:1690–1705.
Chang PC, Wang TY, Chang YT, Chu CY, Lee CL, Hsu HW, et al. Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells. PLoS ONE. 2014;9(2): e88556.
Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, Shen SH, et al. REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling. Oncotarget. 2016;7(18):26137–51.
Quan Z, Li T, Xia Y, Liu J, Du Z, Luo C, et al. PLCɛ maintains the functionality of AR signaling in prostate cancer via an autophagy-dependent mechanism. Cell Death Dis. 2020;11(8):716.
Taniguchi K, Ii H, Kageyama S, Takagi H, Chano T, Kawauchi A, et al. Depletion of gamma-glutamylcyclotransferase inhibits cancer cell growth by activating the AMPK-FOXO3a-p21 axis. Biochem Biophys Res Commun. 2019;517(2):238–43.
Taniguchi K, Matsumura K, Ii H, Kageyama S, Ashihara E, Chano T, et al. Depletion of gamma-glutamylcyclotransferase in cancer cells induces autophagy followed by cellular senescence. Am J Cancer Res. 2018;8(4):650–61.
Ruma IMW, Kinoshita R, Tomonobu N, Inoue Y, Kondo E, Yamauchi A, et al. Embigin promotes prostate cancer progression by S100A4-dependent and-independent mechanisms. Cancers (Basel). 2018;10(7):239.
Grossi V, Lucarelli G, Forte G, Peserico A, Matrone A, Germani A, et al. Loss of STK11 expression is an early event in prostate carcinogenesis and predicts therapeutic response to targeted therapy against MAPK/p38. Autophagy. 2015;11(11):2102–13.
Liao H, Xiao Y, Hu Y, Xiao Y, Yin Z, Liu L, et al. Methylation-induced silencing of miR-34a enhances chemoresistance by directly upregulating ATG4B-induced autophagy through AMPK/mTOR pathway in prostate cancer. Oncol Rep. 2016;35(1):64–72.
Huang Y, Li S, Jia Z, Zhao W, Zhou C, Zhang R, et al. Transient receptor potential melastatin 8 (TRPM8) channel regulates proliferation and migration of breast cancer cells by activating the AMPK-ULK1 pathway to enhance basal autophagy. Front Oncol. 2020;10: 573127.
Zhu G, Wang X, Yang Z, Cao H, Meng Z, Wang Y, et al. Effects of TRPM8 on the proliferation and angiogenesis of prostate cancer PC-3 cells in vivo. Oncol Lett. 2011;2(6):1213–7.
Liu T, Fang Z, Wang G, Shi M, Wang X, Jiang K, et al. Anti-tumor activity of the TRPM8 inhibitor BCTC in prostate cancer DU145 cells. Oncol Lett. 2016;11(1):182–8.
Peng M, Wang Z, Yang Z, Tao L, Liu Q, Yi LU, et al. Overexpression of short TRPM8 variant α promotes cell migration and invasion, and decreases starvation-induced apoptosis in prostate cancer LNCaP cells. Oncol Lett. 2015;10(3):1378–84.
Lin JZ, Wang WW, Hu TT, Zhu GY, Li LN, Zhang CY, et al. FOXM1 contributes to docetaxel resistance in castration-resistant prostate cancer by inducing AMPK/mTOR-mediated autophagy. Cancer Lett. 2020;469:481–9.
Jia J, Zhang HB, Shi Q, Yang C, Ma JB, Jin B, et al. KLF5 downregulation desensitizes castration-resistant prostate cancer cells to docetaxel by increasing BECN1 expression and inducing cell autophagy. Theranostics. 2019;9(19):5464–77.
Li XR, Zhou KQ, Yin Z, Gao YL, Yang X. Knockdown of FBP1 enhances radiosensitivity in prostate cancer cells by activating autophagy. Neoplasma. 2020;67(5):982–91.
Kwon J, Lee Y, Jeong JH, Ryu JH, Kim KI. Inhibition of autophagy sensitizes lignan-induced endoplasmic reticulum stress-mediated cell death. Biochem Biophys Res Commun. 2020;526(2):300–5.
Zhao F, Huang W, Zhang Z, Mao L, Han Y, Yan J, et al. Triptolide induces protective autophagy through activation of the CaMKKβ-AMPK signaling pathway in prostate cancer cells. Oncotarget. 2016;7(5):5366–82.
Jayasooriya R, Dilshara MG, Karunarathne W, Molagoda IMN, Choi YH, Kim GY. Camptothecin enhances c-Myc-mediated endoplasmic reticulum stress and leads to autophagy by activating Ca(2+)-mediated AMPK. Food Chem Toxicol. 2018;121:648–56.
Chang WL, Hsu LC, Leu WJ, Chen CS, Guh JH. Repurposing of nitroxoline as a potential anticancer agent against human prostate cancer: a crucial role on AMPK/mTOR signaling pathway and the interplay with Chk2 activation. Oncotarget. 2015;6(37):39806–20.
Draz H, Goldberg AA, Titorenko VI, Tomlinson Guns ES, Safe SH, Sanderson JT. Diindolylmethane and its halogenated derivatives induce protective autophagy in human prostate cancer cells via induction of the oncogenic protein AEG-1 and activation of AMP-activated protein kinase (AMPK). Cell Signal. 2017;40:172–82.
Tao T, Zhao F, Xuan Q, Shen Z, Xiao J, Shen Q. Fenofibrate inhibits the growth of prostate cancer through regulating autophagy and endoplasmic reticulum stress. Biochem Biophys Res Commun. 2018;503(4):2685–9.
Ben Sahra I, Tanti JF, Bost F. The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy. 2010;6(5):670–1.
Kim SH, Park EJ, Lee CR, Chun JN, Cho NH, Kim IG, et al. Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int J Oncol. 2012;40(5):1683–90.
Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31(8):1424–33.
Yun SM, Jung JH, Jeong SJ, Sohn EJ, Kim B, Kim SH. Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cells. Phytother Res. 2014;28(3):458–64.
Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. Febs j. 2014;281(20):4644–58.
Zhou Z-W, Li X-X, He Z-X, Pan S-T, Yang Y, Zhang X, et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des Devel Ther. 2015;9:1511–54.
Akhtar N, Syed DN, Khan MI, Adhami VM, Mirza B, Mukhtar H. The pentacyclic triterpenoid, plectranthoic acid, a novel activator of AMPK induces apoptotic death in prostate cancer cells. Oncotarget. 2016;7(4):3819–31.
Tang X, Jia J, Li F, Liu W, Yang C, Jin B, et al. Salen-Mn compounds induces cell apoptosis in human prostate cancer cells through promoting AMPK activity and cell autophagy. Oncotarget. 2017;8(49):86277–86.
Bort A, Quesada S, Ramos-Torres Á, Gargantilla M, Priego EM, Raynal S, et al. Identification of a novel 2-oxindole fluorinated derivative as in vivo antitumor agent for prostate cancer acting via AMPK activation. Sci Rep. 2018;8(1):4370.
Younis T, Khan MI, Khan MR, Rasul A, Majid M, Adhami VM, et al. Nummularic acid, a triterpenoid, from the medicinal plant Fraxinus xanthoxyloides, induces energy crisis to suppress growth of prostate cancer cells. Mol Carcinog. 2018;57(10):1267–77.
Liu Y, Wang M, Wang D, Li X, Wang W, Lou H, et al. Malformin A1 promotes cell death through induction of apoptosis, necrosis and autophagy in prostate cancer cells. Cancer Chemother Pharmacol. 2016;77(1):63–75.
Yeung ED, Morrison A, Plumeri D, Wang J, Tong C, Yan X, et al. Alternol exerts prostate-selective antitumor effects through modulations of the AMPK signaling pathway. Prostate. 2012;72(2):165–72.
Díaz-Laviada I, Rodríguez-Henche N. The potential antitumor effects of capsaicin. Prog Drug Res. 2014;68:181–208.
Cheng K, Liu X, Chen L, Lv JM, Qu FJ, Pan XW, et al. α-Viniferin activates autophagic apoptosis and cell death by reducing glucocorticoid receptor expression in castration-resistant prostate cancer cells. Med Oncol. 2018;35(7):105.
Mirkheshti N, Park S, Jiang S, Cropper J, Werner SL, Song CS, et al. Dual targeting of androgen receptor and mTORC1 by salinomycin in prostate cancer. Oncotarget. 2016;7(38):62240–54.
Kumar R, Deep G, Wempe MF, Surek J, Kumar A, Agarwal R, et al. Procyanidin B2 3,3″-di-O-gallate induces oxidative stress-mediated cell death in prostate cancer cells via inhibiting MAP kinase phosphatase activity and activating ERK1/2 and AMPK. Mol Carcinog. 2018;57(1):57–69.
Liu J, Zheng L, Wu N, Ma L, Zhong J, Liu G, et al. Oleanolic acid induces metabolic adaptation in cancer cells by activating the AMP-activated protein kinase pathway. J Agric Food Chem. 2014;62(24):5528–37.
Funding
No particular source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
All the authors declare their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gharibpoor, F., Kamali Zonouzi, S., Razi, S. et al. AMPK’s double-faced role in advanced stages of prostate cancer. Clin Transl Oncol 24, 2064–2073 (2022). https://doi.org/10.1007/s12094-022-02874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02874-z