Abstract
Purpose
Panitumumab plus FOLFOX (P-FOLFOX) is standard first-line treatment for RAS wild-type (WT) metastatic colorectal cancer. The value of panitumumab rechallenge is currently unknown. We assessed addition of panitumumab to FOLFIRI (P-FOLFIRI) beyond progression to P-FOLFOX in patients with no RAS mutations in liquid biopsy (LB).
Methods
In this randomized phase II trial, patients were assigned (3:2 ratio) to second-line P-FOLFIRI (arm A) or FOLFIRI alone (arm B). LB for circulating tumor DNA analysis was collected at study entry and at disease progression. Primary endpoint was 6-month progression-free survival. Two-stage Simon design required 85 patients to be included (EudraCT 2017-004519-38).
Results
Between February 2019 and November 2020, 49 patients were screened (16 RAS mutations in LB detected) and 31 included (18 assigned to arm A and 13 to arm B). The study was prematurely closed due to inadequate recruitment. Serious adverse events were more frequent in arm A (44% vs. 23%). Overall response rate was 33% (arm A) vs. 7.7% (arm B). Six-month progression-free survival rate was 66.7% (arm A) and 38.5% (arm B). Median progression-free survival was 11.0 months (arm A) and 4.0 months (arm B) (hazard ratio, 0.58). At disease progression, RAS or BRAF mutations in LB were found in 4/11 patients (36%) in arm A and 2/10 (20%) in arm B.
Conclusions
The BEYOND study suggests a meaningful benefit of P-FOLFIRI beyond progression to P-FOLFOX in metastatic colorectal cancer patients with WT RAS status selected by LB. This strategy deserves further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) represents 12.7% of all new tumors in the European Union (EU) population [1]. Up to 50% of patients will develop metastases during their evolution. For those with unresectable metastatic disease, treatment is mainly palliative and median survival time is about 30 months. So far, only a few therapeutic agents have shown efficacy in metastatic disease: fluoropyrimidines, oxaliplatin, irinotecan, anti-epidermal growth factor receptor (anti-EGFR) drugs as cetuximab and panitumumab, and anti-angiogenic drugs. For fit patients, they are frequently used upfront in combination, i.e., a chemotherapy regimen plus a targeted agent (according to their molecular profile) [2,3,4]. After progression, second-line regimens include chemotherapy crossover with or without targeted agents. However, treatment options in this setting have limited benefit, and thus, further clinical investigation is warranted.
Activating mutations in KRAS and NRAS are predictive biomarkers of resistance to anti-EGFR monoclonal antibodies in metastatic CRC. In addition, mutations occurring in BRAF, PIK3CA, and PTEN genes have been associated with resistance to these drugs. Panitumumab is labeled in EU in combination with FOLFOX (fluorouracil, folinic acid, and oxaliplatin) in first line (P-FOLFOX) [5] and with FOLFIRI (fluorouracil, folinic acid, and irinotecan) in second line (P-FOLFIRI) [6] for RAS wild-type (WT) patients. The clinical value of anti-EGFR rechallenge strategy (i.e., re-treat with the anti-EGFR in patients previously treated with anti-EGFR and with progressive disease to a second line) has not been definitively demonstrated in randomized clinical trials, although some evidence suggests that it could have potential value [7,8,9,10]. In a somewhat different approach, Ciardello et al. tested for the first time cetuximab continuation plus chemotherapy crossover beyond first progression and showed its potential therapeutic efficacy in molecularly selected patients (KRAS, NRAS, BRAF, and PIK3CA WT status, assessed in baseline tumor biopsy by next-generation sequencing) [7].
Plasma circulating tumor DNA (ctDNA) determination of RAS and BRAF has been shown to correlate well with tissue determination in multiple studies with different techniques [11,12,13,14]. Therefore, continuous monitoring of RAS status is useful during the course of the disease, because it avoids the inconveniences of repeating tumor tissue samples biopsies and is more representative of the current mutational status of the disease. Prospective and retrospective cohort studies suggest that ctDNA RAS mutations detected by liquid biopsy (LB) can identify metastatic colorectal cancer patients with intrinsic or acquired anti-EGFR resistance [15,16,17]. Recently, Cremolini et al. suggested in a retrospective analysis of a prospective phase II clinical trial [18] that a rechallenge strategy with cetuximab benefits specially patients who maintain RAS and BRAF WT status in LB.
We aimed to explore the clinical activity of maintaining panitumumab in combination with FOLFIRI beyond progression to first-line P-FOLFOX in patients with no RAS mutations detected before second-line treatment by LB technology.
Methods
Study design and patients
BEYOND trial (GEMCAD 17-01) is an academic, open-label, randomized phase II trial performed at 15 Spanish hospitals. This trial is registered with EudraCT, no.: 2017-004519-3 8. We enrolled patients aged 18 years or older with histologically confirmed adenocarcinoma of colon or rectum, measurable metastatic disease not amenable to surgical resection, confirmed disease progression to first-line treatment according to RECIST criteria (version 1.1), ECOG performance status of 0–2, and adequate bone marrow, liver and renal function. Patients should have been previously treated in first line with P-FOLFOX, should have achieved complete response (CR), partial response (PR), or stable disease (SD), and have ctDNA WT RAS status determined before randomization. The main exclusion criteria were relevant cardiovascular disease, central nervous system metastases, unresolved toxicities of previous systemic treatment, acute or subacute intestinal occlusion, active inflammatory bowel disease, and major surgery or radiotherapy within 28 days prior to inclusion in the study. The trial was approved by the Ethics Committees at each participating institution and was carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Randomization and treatment
LB for ctDNA analysis was collected at study entry and at disease progression with Idyilla technology. After confirmed progression to first- line P-FOLFOX and having verified the absence of mutations in ctDNA, patients were centrally randomized in a 3:2 ratio to P-FOLFIRI (arm A) or FOLFIRI alone (arm B). Random assignment was stratified by primary tumor sidedness (left vs. right) and, after an amendment dated January 20, 2020, by time since last panitumumab administration (≤ 3 months vs. > 3 months).
On day 1 of each 14-day period, patients in the FOLFIRI group received an infusion of 200 mg l-folinic acid over 1.5–2 h(s), 180 mg per square meter of irinotecan over 1.5 h, 400 mg per square meter bolus of 5-fluorouracil (5-FU) and then 46-h continuous infusion (CI) of 2400 mg per square meter of 5-fluorouracil; patients in the P-FOLFIRI group received an infusion of panitumumab 6 mg per kilogram over 60 min before FOLFIRI. Those patients ≥ 70 years old could start FOLFIRI with a 20% dose reduction. Treatment was continued until disease progression, unacceptable toxic effects, or withdrawal of consent occurred.
Computed tomography imaging was performed at baseline and every 8 weeks until disease progression. Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Adverse events [assessed according to the National Cancer Institute-Common Toxicity Criteria for adverse events (AEs), version 4.03] were recorded continuously.
Statistical analysis
The primary endpoint was 6-month progression-free survival (PFS), defined as the proportion of subjects still alive and progression free at 6 months, and analyzed by intention to treat. The aim of the control arm was to test the validity of the assumption of null effect (6-month PFS of 30%). Sample size was determined through a Simon's two-stage model assuming a null effect corresponding to a 6-month PFS of 30% and a treatment effect corresponding to a 6-month PFS of 50%, with an alpha error of 0.05, and a beta error of 0.2. The sample needed for this study was 46 evaluable subjects in arm A. Considering 10% of possible losses, the final patient number projected to be included was 51 in arm A and 34 in arm B. Under this design, an interim analysis was planned 6 months after the inclusion of the first 15 subjects in arm A and, if the number of patients without progression at 6 months in arm A was less than or equal to 5, the trial would be stopped prematurely because of futility. Analysis of PFS at 6 months was based on the Kaplan–Meier estimator. The survival function as well as the median [95% confidence interval -95% CI-] time to event were estimated by means of the Kaplan–Meier method. Group comparisons were done using the (stratified) log-rank test and the (stratified) hazard ratios (95% CI) were estimated with the Cox model. Secondary analyses were overall response rate (ORR), overall survival (OS), safety and tolerability, as well as biomarker analysis by tissue and LB.
Results
Patients
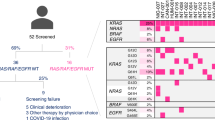
From February 2019 through November 2020, 49 patients were screened for RAS/BRAF/PI3K mutations in ctDNA prior to enrollment. Eighteen patients were excluded by not meeting inclusion/exclusion criteria: sixteen patients because of RAS mutations (32.6%), one patient because not having achieved at least stable disease to first-line P-FOLFOX and one patient due to CNS metastasis at screening. Of 31 eligible patients, 18 were randomized to arm A and 13 to arm B (Fig. 1). Interim analysis was conducted as planned, showing 6 patients without progression 6 months after the inclusion of 15 patients in arm A; therefore, the study continued. However, it was prematurely closed due to low recruitment.
Baseline characteristics were well balanced across study arms (Table 1). There were no major differences in time from the last dose of first-line chemotherapy, time from first-line panitumumab to randomization or in the duration of panitumumab in previous line. Median relative dose intensity for irinotecan, bolus 5-FU, and CI 5-FU was similar in both arms. Median treatment duration and median number of subjects with dose reductions and dose interruptions were higher in arm A. Median number of cycles administered was 12 (Interquartile Range, IQR 7–17) for arm A and 6 (IQR 5–9) for arm B. The only imbalances corresponded to performance status (ECOG PS), with more patients in arm B having ECOG PS 1 (76.9% vs 44.4% in Arm A) and more than one organ affected (100% vs. 55.6%). Altogether, most patients were male, 42% had an ECOG PS of 0, left colon was the primary tumor site in 77.4%, and metastases were located mainly in the liver and lungs. The median duration of follow-up was 9.5 months (IQR 6.0–13.0) with P-FOLFIRI and 7 months (IQR, 5.0–9.0) with FOLFIRI.
Efficacy
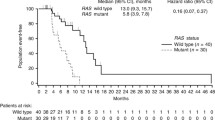
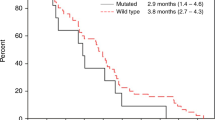
One patient in each arm was not evaluable for response. Confirmed partial tumor responses occurred in 6 patients (33%; 95% confidence interval CI 13–59) in arm A and in 1 patient (7.7%; 95% CI 0.2–36) in arm B. Disease stabilizations were seen in 9 (50%) and 7 (54%) patients, respectively, for a disease control rate of 83% in arm A and 62% in arm B. No complete responses were observed. Best overall response and time of objective response are depicted in Fig. 2. Six-month PFS was 66.7% (95% CI 40.9–86.6) in arm A and 38.5% (95% CI 13.8–68.4) in arm B, corresponding to a hazard ratio (HR) of 0.42, (95% CI 0.14–1.23) (Fig. 3A). The median PFS was 11 months (95% CI 4–14) for arm A and 4 months (95% CI 2–8) for arm B (HR 0.58, 95% CI 0.25–1.3). Median overall survival was 13 months (95% CI 10–20) and 10 months (CI 95% 3–15) in arms A and B, respectively, corresponding to a HR of 0.55 (95% CI 0.2–1.48) (Fig. 3B). At disease progression, mutational status by LB was determined in 21 out of 24 patients, and emergent mutations in RAS were found in 4 out 11 patients (36%) in arm A and in 2 out of 10 patients (20%) in arm B (Table 2). There was a BRAF mutational status change from mutated to wild type in arm B.
Radiologic response. A Waterfall plot of tumor response in evaluable patients, by treatment group. The bars show the best percentage change in the target lesions from baseline. The dashed horizontal lines at 20% and − 30% represent the progressive disease and partial response, respectively. *Patients with progression disease (PD) as best overall response due to new target lesions. B Swimmer plot showing time of objective response (PR or CR) and treatment duration
Adverse events
All patients in the safety population presented at least one adverse event. Overall incidence of grade 3 or 4 adverse events was 66.7% in the P-FOLFIRI group and 53.9% in the FOLFIRI group. The administration of P-FOLFIRI, as compared with FOLFIRI alone, was associated with more asthenia (50% vs. 38.5%), hypomagnesemia (38.9% vs. 7.7%), and acneiform rash (38.9% vs. 0.0%), all of them mainly grade 1–2. With P-FOLFIRI, there was a higher incidence of grade 3 diarrhea (16.7% vs. 7.7%), and in the FOLFIRI arm, there was more frequency of grade 3–4 anemia (15.4% vs 5.6%) (Table 3). Serious AEs were experienced by 44.4% of patients who received P-FOLFIRI compared with 23.1% of patients treated with FOLFIRI. There were two AEs leading to death in both treatment arms (one case in each arm); however, they were not judged as treatment-related by the study investigators.
Discussion
In the continuum of care of metastatic CRC patients treated in first line with chemotherapy plus the anti-vascular endothelial growth factor bevacizumab, it has been shown that a therapeutic option is second-line chemotherapy in combination with anti-angiogenic drugs (bevacizumab, aflibercept, or ramucirumab). It suggests that anti-angiogenic therapy beyond first progression can be effective [21,22,23,24]. For RAS WT patients, anti-EGFR therapy (either alone or in combination with chemotherapy) is considered standard in only a single line of therapy (i.e., first, second, or third) for non-pre-treated patients. More recently, in the setting of anti-EGFR therapy after a first-line anti-EGFR-containing regimen, two different experimental approaches have been explored: (1) the rechallenge, i.e., reintroduction of anti-EGFR therapy after a second-line, anti-EGFR-free schedule; and (2) the continuation of anti-EGFR therapy (plus chemotherapy crossover) beyond progression to an upfront regimen. Both approaches have been assessed with promising results in phase II trials [7, 18, 26]. The underlying hypothesis is that a sustained inhibition of EGFR signaling would continuously eliminate sensitive clones of RAS WT tumor. Pending phase III confirmation, all these studies suggested that the evaluation of RAS mutational status on ctDNA might be helpful in selecting candidate patients. Only patients with RAS and BRAF WT status at relapse determined by LB seem to benefit from these second- or third-line therapies [15].
The BEYOND trial shows a potential benefit of adding panitumumab to FOLFIRI, in comparison with FOLFIRI alone, as second-line treatment for patients with ctDNA WT RAS status. Unfortunately, the estimates are imprecise because of the low number of patients recruited. This is a novel approach evaluating the effects of continuing EGFR inhibition for metastatic CRC beyond tumor progression to a first-line panitumumab-containing treatment. Although the calculated sample size of the study was not met, our results do not rule out a beneficial effect of this re-treatment strategy. Our final figures suggest that we would need 140 patients to be included in a phase III clinical trial to perform a positive study.
The ctDNA study included in our trial confirms data from previous studies [19, 20], indicating that roughly two-thirds of patients receiving anti-EGFR schedules retain their RAS WT status at disease progression. These are accurately the patients that most likely benefit from anti-EGFR re-treatment. ORR (between 6 and 10%) and PFS (between 4 and 5 months) in our control arm are within the range of those reported in the literature with second-line FOLFIRI [21,22,23] or FOLFIRI plus bevacizumab [24] and for the RAS naïve enriched population treated with FOLFIRI [6]. Therefore, the observed differences are unlikely to arise from a worse outcome in the control arm. We decided to use FOLFIRI alone, instead of FOLFIRI plus bevacizumab in the control arm, because there is no clinical evidence that bevacizumab provides benefit compared to FOLFIRI based on phase III clinical trials after first-line P-FOLFOX. Recently, a prospective clinical trial comparing second-line FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab in RAS WT (based on baseline tumor tissue determination) metastatic CRC patients previously treated with FOLFOX plus cetuximab did not demonstrate cetuximab benefit in response rate or PFS [25], but suggested that only patients that retain RAS WT status before second-line therapy would benefit from rechallenge strategy [18]. Our ORR (33%; 95% CI 13–59%) and PFS (11 months; 95% CI 4–14 months) in the experimental arm are in line with that obtained with second-line P-FOLFIRI in FOLFOX only pre-treated patients [6]. Recently, the CHRONOS study [26] with a rechallenge strategy showed similar ORR (30%, 95% CI 12–47%) than that achieved with panitumumab monotherapy in previously untreated patients [27].
Despite initial benefit in the P-FOLFIRI arm, most patients included in the study progressed. Interestingly, at the time of disease progression, only 36% of patients in the P-FOLFIRI group showed emergent RAS mutations in LB, which is in the range of other studies [28]. We have observed RAS emergent mutations in 20% of patients of the FOLFIRI control arm, suggesting that acquisition of RAS mutations could appear also on holding anti-EGFR periods. All these data confirm that alternative driven mutations in EGFR, PIK3CA, AKT, or ERBB2, MET amplification [29,30,31,32,33,34], or transcriptomic mechanisms of resistance [35] play a critical role in intrinsic and acquired anti-EGFR resistance.
The safety profile of P-FOLFIRI treatment was in line with that expected. The incidence rate of grade 3 or diarrhea and acne-like rash reactions were higher with P-FOLFIRI than with FOLFIRI alone, as well as the overall grade 3 or 4 adverse events. However, these adverse events were generally manageable. Regrettably, we did not performed quality of life questionnaires that would have better assessed the clinical impact of this treatment.
In conclusion, the BEYOND study suggests a meaningful clinical benefit of adding panitumumab to FOLFIRI beyond progression to first-line P-FOLFOX in metastatic CRC patients with WT RAS status selected by LB at relapse. More globally, it also supports the potential value of these novel approaches evaluating anti-EGFR re-treatment (continuation or rechallenge) in the second- or third-line treatment. Although the study was closed prematurely, this strategy deserves further investigation.
References
Cancer incidence and mortality in EU-27 countries. https://ec.europa.eu/jrc/en/news/202-cancer-incidence-and-mortality-eu-27-countries. Accessed 10 May 2022.
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37.
Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866–75.
Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. 2016;24:1539–46.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) Versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705.
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:107–16.
Ciardiello F, Normanno N, Martinelli E, Troiani T, Pisconti S, Cardone C, et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol. 2016;27:1055–61.
Feng Q, Wei Y, Ren L, Zheng P, Yu Y, Ye Q, et al. Efficacy of continued cetuximab for unresectable metastatic colorectal cancer after disease progression during first-line cetuximab-based chemotherapy: a retrospective cohort study. Oncotarget. 2016;7:11380–96.
Liu X, George GC, Tsimberidou AM, Naing A, Wheler JJ, Kopetz S, et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer. 2015;15:343–50.
Santini D, Vincenzi B, Addeo R, Garufi C, Masi G, Scartozzi M, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23:2313–8.
Maurel J, Alonso V, Escudero P, Fernández-Martos C, Salud A, Méndez M, et al. Clinical impact of circulating tumor RAS and BRAF mutation dynamics in patients with metastatic colorectal cancer treated with first-line chemotherapy plus anti-epidermal growth factor receptor therapy. JCO Precis Oncol. 2019;3:1–16.
Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017;28:1294–301.
Schmiegel W, Scott RJ, Dooley S, Lewis W, Meldrum CJ, Pockney P, et al. Blood-based detection of RAS mutations to guide anti-EGFR therapy in colorectal cancer patients: concordance of results from circulating tumor DNA and tissue-based RAS testing. Mol Oncol. 2017;11:208–19.
Vidal J, Muinelo L, Dalmases A, Jones F, Edelstein D, Iglesias M, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol. 2017;28:1325–32.
Normanno N, Esposito Abate R, Lambiase M, Forgione L, Cardone C, Iannaccone A, et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol. 2018;29:112–8.
Siena S, Sartore-Bianchi A, Garcia-Carbonero R, Karthaus M, Smith D, Tabernero J, et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol. 2018;29:119–26.
Cremolini C, Morano F, Moretto R, Berenato R, Tamborini E, Perrone F, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28:3009–14.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–50.
Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506.
Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind study. Lancet Oncol. 2015;16:499–508.
André T, Louvet C, Maindrault-Goebel F, Couteau C, Mabro M, Lotz JP, et al. CPT-11 (Irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. Eur J Cancer. 1999;35:1343–7.
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–33.
Li D, Wang F, Xu S, Li K, Meng X, Huang Y, et al. Continuing Cetuximab vs Bevacizumab plus chemotherapy after first progression in wild-type KRAS, NRAS and BRAF V600E metastatic colorectal cancer: a randomized phase II trial. J Cancer. 2021;12:5268–74.
Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Fenocchio E, et al. Phase II study of anti-EGFR rechallenge therapy with panitumumab driven by circulating tumor DNA molecular selection in metastatic colorectal cancer: the CHRONOS trial. J Clin Oncol. 2021;39(15 suppl):3506.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34.
Kim TW, Peeters M, Thomas A, Gibbs P, Hool K, Zhang J, et al. Impact of emergent circulating tumor DNA Ras mutation in panitumumab-treated chemoresistant metastatic colorectal cancer. Clin Cancer Res. 2018;24:5602–9.
Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the prospect-c phase ii colorectal cancer clinical trial. Cancer Discov. 2018;8:1270–85.
Morelli MP, Overman MJ, Dasari A, Kazmi SMA, Mazard T, Vilar E, et al. Characterizing the patterns of clonal selection in circulating tumor DNA from patients with colorectal cancer refractory to anti-EGFR treatment. Ann Oncol. 2015;26:731–6.
Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:2414–22.
Xu JM, Wang Y, Wang YL, Wang Y, Liu T, Ni M, et al. PIK3CA mutations contribute to acquired cetuximab resistance in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:4602–16.
Toledo RA, Cubillo A, Vega E, Garralda E, Alvarez R, de la Varga LU, et al. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget. 2017;8:35289–300.
Goldberg RM, Montagut C, Wainberg ZA, Ronga P, Audhuy F, Taieb J, et al. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open. 2018;3:e000353. https://doi.org/10.1136/esmoopen2018-000353.
Woolston A, Khan K, Spain G, Barber LJ, Griffiths B, Gonzalez-Exposito R, et al. Genomic and transcriptomic determinants of therapy resistance and immune landscape evolution during anti-EGFR treatment in colorectal cancer. Cancer Cell. 2019;36:35-50.e9.
Funding
This work was supported by AMGEN S.A.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dr J. Aparicio received honoraria for consultant or advisory role for Amgen, Merck, Sanofi, Servier, Bayer and Pierre Fabre. Dr A.C. Virgili reported serving on speakers´ bureaus for Amgen, Sanofi, and Servier; received honoraria for scientific advisory role from Roche and Amgen; and receiving travel grants from Merck, Roche, Amgen, Sanofi, MSD, and Servier. Dr J. Capdevila reported receiving honoraria for serving on speakers´ bureaus and scientific advisory from Amgen, Bayer, AAA, AstraZeneca, Eisai, Exelixis, Ipsen, ITM, Hudchinson Pharma, Lilly, Novartis, Pfizer, and Sanofi; and receiving research grants from AAA, Eisai, Ipsen, Novartis, and Pfizer. Dr D. Paez reported receiving honoraria from Amgen, Sanofi, and Novartis; for serving in advisory roles from Amgen, Ipsen, and Servier; reported receiving research funding from Merck; and reported receiving travel grants from Amgen, Merck, Roche, Lilly, Servier, Sanofi, and Ipsen. Dr J. Hernando reported receiving honoraria from speakers´ bureaus from Ipsen, Eisai, Novartis, Adacap, Leo, Angelini, Roche, and Pfizer. Dr R. Vera received consultant fees from Roche, Amgen, Merck, Sanofi, and Bristol Myer Squibb; and received advisory honorarium from Roche, Amgen, Merck Sharp, and Dohme and Sanofi. Dr X. Hernandez-Yagüe reported receiving honoraria for serving in an advisory role from Sanofi, Amgen, and Merck. Dr J. Maurel received research grants from Merck, Roche, Amgen, NanoString, Incyte, and Biocartis; reported personal fees from Advance Medical, Cancer Expert Now, Fundación Clínica Universitaria, Sirtex, Pierre-Fabre, Shire, Astra-Zeneca, Bayer, Servier, Sanofi, and Roche; and received grants from Catalan Agency for Management of University and Research Grants (AGAUR) (2014-SGR-474 and 2017-SGR-1174), Fundació la Marató de TV3 (201330.10), Instituto de Salud Carlos III (PI13/01728 and PI19/00740), Fundacion Olga Torres (Modalitat A. 2019/2020), and Spanish Association Against Cancer (AECC, PROYE19040POST_001). All remaining authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aparicio, J., Virgili Manrique, A.C., Capdevila, J. et al. Randomized phase II trial of FOLFIRI-panitumumab compared with FOLFIRI alone in patients with RAS wild-type circulating tumor DNA metastatic colorectal cancer beyond progression to first-line FOLFOX-panitumumab: the BEYOND study (GEMCAD 17-01). Clin Transl Oncol 24, 2155–2165 (2022). https://doi.org/10.1007/s12094-022-02868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02868-x