Abstract
Background and purpose
Synchronous bilateral breast cancer (SBBC) accounts for 1–3.5% of breast cancer patients. The aim of this study was to evaluate dosimetric issues, clinical outcomes, and acute toxicities for SBBC patients receiving synchronous bilateral hypofractionated radiotherapy (SBHRT) and to compare them with patients treated with synchronous bilateral normofractionated RT schedule (SBNRT).
Materials and methods
From April 2016 to March 2020, 39 SBBC patients were referred to our institution. Patients were divided according to their prescription dose: Group A: 50 Gy/25fx (fractions), B: 60–64 Gy/25fx, C: 40.05 Gy/15fx; D: 48 Gy/15fx. Toxicity was evaluated using Common Terminology Criteria for Adverse Events (CTCAE)v.5.0.
Results
34 patients were finally evaluated. Median follow-up was 24 months for NF schedule and 9 months for HF schedule. In the HF schedule, no acute side-effects > G2 were observed and no dermatitis was reported in 6th month´s assessments. 95% of patients have no evidence of disease and only 1 patient presented local relapse in the first mammography after RT. No distant failures or deaths were observed. Regarding dosimetric issues, the inter-patient average Dmean for the heart was: Group A: 5.0 Gy (4.6–5.5), Group B: 4.4 Gy (4.1–5.4), Group C: 4.8 Gy (4.5–5.1) and Group D: 5.3 Gy (4.4–5.6). For the lungs, the inter-patient average Dmean was: Group A: 10.8 Gy (9.8–12.2), Group B: 11.5 Gy (11.3–12), Group C: 9.8 Gy (9.3–10.5) and Group D: 10.5 Gy (10–11.3).
Conclusions
This is the first study reporting the safety, feasibility, and tolerability of 40.05 Gy/15fx over 3 weeks for the treatment of SBBC patients. Further study with larger accrual is mandatory.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the 2nd most common cancer worldwide and the 1st cause of death from cancer in developed countries [1]. In fact, 12.5% of women will develop breast cancer during their life [2]. Synchronous bilateral breast cancer (SBBC) is defined as two malignant tumors diagnosed within an interval of 6 months one in each breast [3]. This uncommon condition accounts for 1–3.5% of all BC patients [4]. Nevertheless, SBBC is not yet demonstrated as a worse prognosis than unilateral BC. According to some studies, synchronous bilaterality was not an independent prognostic risk factor on multivariate analysis when compared to unilateral BC [5, 6].
With the development of modern irradiation techniques, such as Intensity Modulated Radiation Therapy (IMRT) and Volumetric Arc Radiation Therapy (VMAT), some authors have demonstrated the feasibility, tolerability, and safety of such techniques when treating both breasts at the same time in SBBC patients [3, 4]. However, all patients in these studies were treated following normofractionated (NF) radiotherapy (RT) schedule of 50 Gy in 25 fractions (fx) with or without simultaneously integrated boost (SIB) up to 60 Gy. In 2013, the 10-year follow-up results of the START trials demonstrated that hypofractionated radiotherapy (HF) was safe and effective for patients with early breast cancer, supporting the use of 40.05 Gy in 15fx as the standard of care for women requiring adjuvant radiotherapy for invasive early breast cancer [7]. Since then, this RT schedule is increasingly being adopted by radiation oncology departments worldwide. However, the use of synchronous bilateral hypofractionated radiotherapy (SBHRT) in SBBC patients has not yet been published in the literature.
The aim of this observational prospective study was to evaluate dosimetric and clinical outcomes in SBBC patients treated with SBHRT and to compare them to those obtained with the classical synchronous bilateral normofractionated radiotherapy (SBNRT) schedule.
Materials and methods
Patient selection
From June 2016 to March 2020 1234 patients with diagnosed breast cancer were referred to our institution to receive RT. 39 (3.16%) of these patients had SBBC and were enrolled in our study. Of the 9 women prescribed chemotherapy, 6 (66%) received it in neoadjuvant and 3 (33%) in adjuvant setting. In both cases, the treatment consisted in 4 anthracycline cycles followed by 12 taxane cycles. In HER 2 + cases, the patients received trastuzumab + pertuzumab + chemotherapy before the surgery and maintained trastuzumab for a year. All patients except 1 (double triple negative) received adjuvant hormonal therapy depending on the menopausal status. The study was approved by the internal review board and every patient signed an individual informed consent.
Target volume and OAR delineation
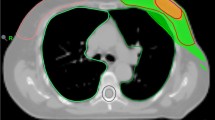
Patients were immobilized in a supine position and a planning computed tomography (CT) scan (General Electric OPTIMA 580, GE Healthcare, Milwaukee, WI, USA) with no intravenous contrast was acquired using 2.5 mm thick slices. The planning system used was ECLIPSE/ARIA (Eclipse Planning System v13, Varian Medical Systems, Palo Alto, CA, USA), which includes a photon optimizer (PO) inverse planning algorithm and the plans were calculated with Analytical Anisotropic Algorithm (AAA) v13. The clinical target volume of the breast (CTV breast) was outlined including all breast tissue (85%) or chest wall (15%) and lymph node levels I-IV if clinically indicated. The clinical target volume of the surgical bed (CTV boost) was contoured for patients who met any of the following risk factors: < 50 years old, Grade 3 (G3) ductal in situ or ductal/lobular invasive carcinoma or positive margins after surgery. The volume included visible surgical clips, seroma cavity, and anatomical distortions. Both planning target volumes (PTV) breast and PTV boost were generated adding a 5 mm isotropic expansion to CTV breast and CTV boost respectively and cropping 5 mm to PTV extending outside the body. According to the Organs at Risk (OARs), heart, lungs, esophagus and spinal cord were considered. Target volumes and OARs were delineated following the ESTRO (European Society of Radiation Oncology) recommendations [8].
RT dose and delivery
Patients were divided into two main dose groups. On the one hand, 14 patients were treated with SBNRT delivering 50 Gy in 25fx to PTV breast. When a simultaneously integrated boost (SIB) was needed, these patients received 60 Gy in 25fx to PTV boost. On the other hand, 20 patients received SBHRT to PTV breast administering 40.05 Gy in 15fx with a SIB of 48 Gy in 15fx to PTV boost when indicated. SIB was administered if the patient met any of the following conditions: Age < 60, Grade 3 tumor, lymphovascular invasion or positive margins.
Patients were treated using VMAT technique with 6MV photons generated by a Varian Clinac DHX accelerator, equipped with a Millennium 120-leaf collimator (MLC) and an On-Board-Imager for image-guided radiation therapy IGRT (Varian Medical Systems, Palo Alto, CA, USA) (Fig. 1). Plans were approved by radiation oncologists if 100% isodose covered more than 95% of target PTVs with a D2% no higher than 110% of the prescription dose.
According to OARs, different constraints were considered depending on the prescribed dose and fractionation. In the SBNRT group heart limits were: Dmean < 7.5 Gy, V20 < 5% and V10 < 30%. Lungs limits were: both lungs Dmean < 15 Gy, V20 < 15% and V10 < 40%. In the SBHRT group heart limits were: Dmean < 5 Gy, V25 < 10% and V8 < 35%. Lungs limits were: both lungs Dmean < 15 Gy, V20 < 20% and V10 < 40%. The constraints on the OARs for planning approval were those in our department protocol derived from the RTOG 1005 study. However, as these constraints are designed for unilateral breast cancer, Dmean constraints for the heart and lungs were rescaled for the planning of bilateral breast cancer [9].
During the treatment IGRT was performed for the first five days and then once weekly until the end of the treatment with a cone-beam computerized tomography (CBCT).
Disease control and toxicity recording
Treatment-related acute toxicity was recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) 5.0 criteria [10]. The observed toxicity in patients previous to 2017 was re-interpreted according to this scale. Patients were followed up systematically weekly during treatment and every time the patient asked for medical visitation. Patients were cited 1 month and 6 months after finishing RT treatment. All the existing toxicities were reported including skin dermatitis, skin hyperpigmentation, and esophagitis.
Regarding disease control, mammography was performed once annually and the first one at least 6 months after finishing RT. If disease progression was suspected, bone scintigraphy, a complete blood test with tumor markers, and computed tomography (CT) were requested. According to the results, patients were divided into 4 categories: No evidence of disease (NED), loco-regional failure, distant failure, and death.
Statistical analysis
A descriptive analysis of all variables has been carried out to define the characteristics of the study group with frequencies and percentages for the qualitative variables and with measures of central position and dispersion for the quantitative variables. Variables with normal distribution are expressed by means and standard deviation, and non-normal variables by median and interquartile range. To evaluate the differences between groups, the student's T test (in case of normality) and the U-Mann–Whitney test (in case of non-normality) were applied for quantitative variables and the chi-square test or exact test of Fisher for qualitative variables.
Statistical analysis was performed using the software IBM-SPSS v.26. A p value of < 0.05 was considered statistically significant.
Results
Among the 39 reviewed SBBC patients, only 34 were finally evaluated. 3 of these patients were not able to receive RT, 2 of them due to Anthracycline-related cardiotoxicity and 1 due to chemotherapy (CT) related septic shock. Another patient received palliative RT and 1 patient received RT only to the left breast so they were therefore excluded. The mean age of the treated patients was of 65 years old with a range from 37 to 82. Patient characteristics are shown in Table 1.
Acute toxicity
There was only 1 case of G3 toxicity reported in the first 6 months after RT. It was 1 patient treated in the SBNRT group that had G3 dermatitis during RT and the first month after treatment. However, this was completely resolved at the 6th month’s assessment. No other G3 or superior acute toxicities were observed in any group. Complete details are shown in Table 2.
Clinical outcomes
The median follow-up for the NF schedule and the HF schedule was 24 months (1–36) and 9 (6–42) months respectively.
In the SBNRT group, 11 patients (79%) have NED 2 years after RT. 1 patient presented distant failure with bone metastases 10 months after finishing RT and is alive with disease. 2 patients died during the follow-up, 1 of them due to a respiratory failure after a broncho-aspiration and the other patient, who presented multiple bone metastases at the diagnosis, died because of disease progression.
In the SBHRT group 19 patients (95%) were free from disease. 1 patient (5%) presented local relapse in the first mammography at 6 months after finishing RT. No distant failures or deaths were observed in this group.
Dosimetric results
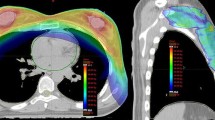
The values of the conceived dose-volume metrics for the OARs are reported in Table 3 for the SBNRT group (Group A) and SBNRT + SIB group (Group B). Furthermore, the same items can be observed for the SBHRT group (Group C) and SBHRT + SIB (Group D) in Table 4.
In addition, a comparison of Dmean for the OARs between both treatment schedules with and without boost are reported in Table 5.
Discussion
Synchronous bilateral breast cancer irradiation represents a challenge in the current clinical practice due to the large target volume and the need to minimize the dose to critical OARs such as the heart, lungs, esophagus, and spinal cord. Although BC represents a large volume of work in radiation oncology departments, SBBC is a rare disease and clinical guidelines for SBBC irradiation are lacking. The development of modern radiation techniques such as IMRT, VMAT and helical tomotherapy (HT) allows us to treat SBBC patients with a better target dose homogeneity and sparing of OARs.
Several recently published studies have discussed which radiation technique was the best for SBBC irradiation according to dosimetric characteristics. Cheng et al. compared the dosimetric characteristics of HT, VMAT, IMRT, and tangential field-in-field techniques for the treatment of SBBC. The authors concluded that HT provided the most favorable dose sparing of OARs, although it required a longer beam-on time which could result in an increase in patient discomfort [11]. Cho et al. evaluated the optimal RT plan for 15 SBBC patients including regional LN. According to their results, a modified hybrid plan using VMAT + modified 3D-CRT is the best when considering both PTV coverage and protection of OARs [12]. In contrast, Kim et al. compared IMRT, VMAT, and 3D-CRT concluding that IMRT was superior to the other techniques in terms of dose distribution [13]. Finally, Huang et al. investigated the fixed-jaw IMRT (F-IMRT) and tangential partial VMAT (tP-VMAT) treatment plans for SBBC patients. They assessed that both techniques were of high quality and feasible for SBBC patients [14].
For our SBBC patients, VMAT plans using 4 partial coplanar arcs resulted in adequate target dose coverage with a satisfactory dose sparing to critical OARs. Regarding dose coverage, the average D2% was no higher than 110% and the 100% isodose covered at least 95% of target PTVs in all the studied patients. According to the Heart, Darby et al. conducted a population-based case–control study of major coronary events in 2168 women who underwent radiotherapy for breast cancer. They reported that a Dmean of 3-4 Gy to the heart was an acceptable value for unilateral breast irradiation and that rates of major coronary events increased linearly with the mean dose to the heart by 7.4% per Gy [15]. In our study, Dmean to the heart was around 5 Gy for both SBNRT and SBHRT groups which results in an acceptable value for bilateral breast irradiation and is consistent with other studies reporting cardiac toxicity. Fiorentino et al. published a study with 16 women with SBBC treated with VMAT with 50 Gy in 25fx. Their reported inter-patient average Dmean for the heart was 8.3 ± 3.3 Gy [4]. In a recently published study, Sun et al. identified from their database 11 patients with SBBC that had received RT and designed different treatment plans using IMRT, VMAT, HT and intensity-modulated proton therapy (IMPT). Their mean objective was to compare heart and cardiac substructures such as left ventricle (LV) and left anterior descending artery (LAD) dose sparing comparing photon and proton RT. According to their results, the IMPT plan showed the lowest values for the V5, V10, V20 and Dmean for the Heart, LV and LAD (p < 0.05, p < 0.05 and p < 0.01 respectively). Although a Dmean < 1 Gy was achieved using IMPT, it is still a less common and more expensive technique that cannot currently be taken as the standard of care for SBBC patients. In addition, skin toxicities seemed to be greater with proton therapy comparing with photon therapies [16].
For lungs, a Dmean < 13 Gy and V20 < 30% were recommended in order to decrease the risk for radiation pneumonitis (RP). In our study, we proposed even more strict constraints achieving a Dmean for both lungs of around 11 Gy and an average V20 of 12–14% with both RT schedules. These results are comparable to those reported by Fiorentino with a Dmean of 11.8 ± 2.3 Gy and a V20 of 15.7 ± 5% [4]. Seppala et al. reported similar dosimetric findings for the lungs (Dmean 10 Gy and V20 of 17%) although only 2 cases were analyzed [17]. Valli et al. published a study with 25 SBBC patients treated with VMAT using a NFRT schedule with the aim of reporting skin and lung toxicities during and after the treatment. Their reported G1 and G2 dermatitis rates of 72% and 24% respectively during the RT. No symptomatic RP was observed during RT, at 6 weeks or at 6 months after the end of RT [3].
Several studies have reported different outcomes using RT for SBBC, but as far as we know, this is the first study reporting clinical, dosimetric and toxicity outcomes for SBBC patients following an HFRT schedule. Due to its radiobiological characteristics, it has been strongly demonstrated that breast cancer patients can benefit from hypofractionated radiation schedules. Breast tumor´s α/β ratio is considered to be similar or inferior to the ratio of its surrounding normal tissues. If late normal tissue effect α/β ratios exceed the tumor α/β ratio, hypofractionation widens the therapeutic ratio, providing lower late toxicity at a constant tumor BED [18].
The first studies comparing standard fractionation with hypofractionated schedules were designed in 2008 by the START´s trialists group. The START A and START B trials compared different hypofractionated schedules with the classical 50 Gy in 25fx schedule in terms of loco-regional tumor control and late normal tissue effects [19, 20]. The 10-year follow-up results confirmed that HF radiotherapy was safe and effective for early breast cancer and supported the use of 40 Gy in 15fx as the standard of care for women requiring adjuvant radiotherapy for invasive early breast cancer [7]. Whelan et al. [21] conducted a study including women with invasive breast cancer who had undergone BCS with negative surgical margins and negative axillary lymph nodes and were randomized to either NF or HF schedules. In the 10-year follow-up HF schedule appeared to be not inferior to the NF schedule in terms of local control and cosmetic outcomes. A recently published study with more than 12 years of follow-up confirmed that modest HF provides better breast cancer-specific outcomes compared with NF schedules also in nodal positive patients [22].
Taking the above reported into account, we analyzed clinical outcomes and acute toxicity in a cohort of SBBC patients treated with an HFRT schedule. According to our results, 95% of patients treated with the HFRT schedule are free of disease and no distant failure or deaths have been observed in this group. These results are similar to the clinical outcomes reported by other authors with the NFRT schedule [4]. In reference to acute toxicity, both RT schedules showed acceptable dermatitis rates, with no G2-G3 cases observed at the 6-month assessment with none of the RT schedules. There were only a few G1 esophagitis cases that were resolved with non-steroidal anti-inflammatory drugs (NSAIDs) and G1 skin hyperpigmentation appeared by the 6-month assessment in 1 patient and 3 patients treated with HFRT and NFRT respectively. No esophagitis G3 or skin hyperpigmentation G2 cases were observed. In contrast with these results, Kaider-Person et al. showed a significant RT toxicity in SBBC treated with HT. They included 9 patients with locoregional nodal involvement reporting high rates of skin desquamation, dysphagia, and fatigue. In the present analysis, LNs were included in the target PTV in 30% of the cases and we did not observe any major toxicity in such cases [23].
The present study demonstrates that 40.05 Gy in 15fx is a safe and feasible RT schedule for SBBC patients with or without nodal involvement in terms of clinical outcomes, dosimetric issues, and acute toxicity. However, it is logical to think that moderate hypofractionation is not the limit. It is a fact that there is a tendency towards increasingly extreme hypofractionation as it provides clear benefits for patients, Radiation Oncology departments, and health systems [24]. In addition, with the current Sars-Cov-2 pandemic, extreme hypofractionation would reduce the visits of the patients to the hospital reducing the risk of infecting and being infected with Sars-Cov-2 and therefore decreasing the risk of suffering the serious consequences this disease entails. Focusing on BC patients, in 2020 5-year efficacy and late normal tissue effects from the FAST-Forward study have been reported. FAST-Forward is a phase III, multicentre, non-inferiority, randomized controlled trial that aims to identify a 1 week, (5fx) schedule of curative radiotherapy that is at least as effective and safe as the current standard 15 fraction regimen. According to their results, 26 Gy in 5fx over 1 week is non-inferior to the standard HF schedule for local tumor control and is as safe in terms of normal tissue effects up to 5 years after RT [25, 26]
Conclusion
To our knowledge, this is the first study reporting the safety, feasibility, and tolerability of 40.05 Gy in 15fx over 3 weeks for the treatment of SBBC patients. Although a longer follow-up is needed, HFRT is eligible for the treatment of SBBC patients as it is established for unilateral breast cancer.
Availability of data and materials
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Code availability
Not applicable.
References
Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. https://doi.org/10.1007/s13304-017-0424-1.
Oncogía SEGO: Carcinoma infiltrante de mama 2017. https://www.semnim.es/wp-content/uploads/2019/07/349.pdf. Accessed 28 Nov 2020.
Valli M, Cima S, Gaudino D, Cartolari D, Deantonio L, Frapolli M, et al. Skin and lung toxicity in synchronous bilateral breast cancer treated with volumetric-modulated arc radiotherapy: a mono-institutional experience. Clin Transl Oncol. 2019;21(11):1492–8. https://doi.org/10.1007/s12094-019-02077-z.
Fiorentino A, Mazzola R, Naccarato S, Giaj-Levra N, Fersino S, Sicignano G, et al. Synchronous bilateral breast cancer irradiation: clinical and dosimetrical issues using volumetric modulated arc therapy and simultaneous integrated boost. Radiol Med. 2017;122(6):464–71. https://doi.org/10.1007/s11547-017-0741-y.
Carmichael AR, Bendall S, Lockerbie L, Prescott R, Bates T. The long-term outcome of synchronous bilateral breast cancer is worse than metachronous or unilateral tumours. Eur J Surg Oncol. 2002;28:388–91. https://doi.org/10.1053/ejso.2002.1266.
Mose S, Adamietz IA, Thilmann C, Saran F, Bernhard M, Pahnke R, Böttcher HD. Bilateral breast carcinoma versus unilateral disease. Review of 498 patients. Am J Clin Oncol. 1997;20(6):541–5. https://doi.org/10.1097/00000421-199712000-00001.
Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, START Trialists’ Group, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–94. https://doi.org/10.1016/S1470-2045(13)70386-3.
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3–10. https://doi.org/10.1016/j.radonc.2014.11.030.
A phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer protocol. https://www.nrgoncology.org/Clinical-Trials/Protocol/rtog-1005?filter=rtog-1005. Accessed 12 Mar 2021.
U.S. Department of Health and Human Services. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf. Accessed 29 Nov 2020.
Cheng HW, Chang CC, Shiau AC, Wang MH, Tsai JT. Dosimetric comparison of helical tomotherapy, volumetric-modulated arc therapy, intensity-modulated radiotherapy, and field-in-field technique for synchronous bilateral breast cancer. Med Dosim. 2020;45(3):271–7. https://doi.org/10.1016/j.meddos.2020.01.006.
Cho Y, Cho YJ, Chang WS, Kim JW, Choi WH, Lee IJ. Evaluation of optimal treatment planning for radiotherapy of synchronous bilateral breast cancer including regional lymph node irradiation. Radiat Oncol. 2019;14(1):56. https://doi.org/10.1186/s13014-019-1257-5.
Kim SJ, Lee MJ, Youn SM. Radiation therapy of synchronous bilateral breast carcinoma (SBBC) using multiple techniques. Med Dosim. 2018;43:55–68. https://doi.org/10.1016/j.meddos.2017.08.003.
Huang JH, Wu XX, Lin X, Shi JT, Ma YJ, Duan S, Huang XB. Evaluation of fixed-jaw IMRT and tangential partial-VMAT radiotherapy plans for synchronous bilateral breast cancer irradiation based on a dosimetric study. J Appl Clin Med Phys. 2019;20(9):31–41. https://doi.org/10.1002/acm2.12688.
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. https://doi.org/10.1056/NEJMoa1209825.
Sun T, Lin X, Tong Y, Liu X, Pan L, Tao C, et al. Heart and cardiac substructure dose sparing in synchronous bilateral breast radiotherapy: a dosimetric study of proton and photon radiation therapy. Front Oncol. 2020;10(9):1456. https://doi.org/10.3389/fonc.2019.01456.
Seppälä J, Heikkilä J, Myllyoja K, Koskela K. Volumetric modulated arc therapy for synchronous bilateral whole breast irradiation: a case study. Rep Pract Oncol Radiother. 2015;20:398–402. https://doi.org/10.1016/j.rpor.2015.05.011.
Brand DH, Yarnold JR. The Linear-Quadratic Model and implications for fractionation. Clin Oncol (R Coll Radiol). 2019;31(10):673–7. https://doi.org/10.1016/j.clon.2019.06.007.
Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9:331–41. https://doi.org/10.1016/S1470-2045(08)70077-9.
Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet. 2008;371:1098–107. https://doi.org/10.1016/S1470-2045(13)70386-3.
Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20. https://doi.org/10.1056/NEJMoa0906260.
Koulis TA, Nichol AM, Truong PT, Speers C, Gondara L, Tyldesley S, et al. Hypofractionated adjuvant radiation therapy is effective for patients with lymph node-positive breast cancer: a population-based analysis. Int J Radiat Oncol Biol Phys. 2020;108(5):1150–8. https://doi.org/10.1016/j.ijrobp.2020.07.2313.
Kaidar-Person O, Kostich M, Zagar TM, Jones E, Gupta G, Mavroidis P, et al. Helical tomotherapy for bilateral breast cancer: clinical experience. Breast. 2016;28:79–83. https://doi.org/10.1016/j.breast.2016.05.004.
Ray KJ, Sibson NR, Kiltie AE. Treatment of breast and prostate cancer by hypofractionated radiotherapy: potential risks and benefits. Clin Oncol. 2015;27:420–6. https://doi.org/10.1016/j.clon.2015.02.008.
FAST-Forward_protocol v5.1 5 Feb 2018. https://d1ijoxngr27nfi.cloudfront.net/docs/default-source/default-document-library/fast-forward-protocol.pdf?sfvrsn=421a2169_0. Accessed 28 Nov 2020.
Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield AJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet Oncol. 2020;395(10237):1613–26. https://doi.org/10.1016/S0140-6736(20)30932-6.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jon Gadea Quinteiro, Irene Ortiz Gonzalez and Raquel Roncero Sanchez. The first draft of the manuscript was written by Jon Gadea Quinteiro and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publications
The participant has consented to the submission of the original article to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gadea, J., Ortiz, I., Roncero, R. et al. Synchronous bilateral breast cancer treated with a 3-week hypofractionated radiotherapy schedule: clinical and dosimetric outcomes. Clin Transl Oncol 23, 1915–1922 (2021). https://doi.org/10.1007/s12094-021-02600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02600-1