Abstract
Purpose
To evaluate the added value of diffusion-weighted imaging (DWI) to T2-weighted imaging (T2WI) for improved identification of pelvic lymph nodes (LN) by radiation oncologists.
Methods/patients
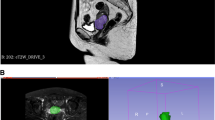
This retrospective study included 20 patients with histopathologically proven node-negative prostate cancer. All patients underwent 3T-MRI of the prostate; matched axial T2WI and DWI sequences were assessed by an experienced uro-radiologist as the reference standard. Consultant and specialist registrar radiation oncologists were asked to identify all LN first on T2WI alone (read 1) and then on T2WI and DWI combined (read 2); LN were measured in size and divided into true positives (TP), false positives (FP) and false negatives (FN). Sensitivity, positive predictive value (PPV) and false negative rate (FNR) were then calculated and compared using Pearson’s Chi square test.
Results
A total of 177 LN comprised the reference standard. 16 TP, 16 FP and 161 FN LN (sensitivity 9.0%, PPV 50.0%, FNR 91.0%) and 124, 15 and 53 LN (70.1%, 89.2%, 30%) were identified by reader 1 on reads 1 and 2, respectively; χ2 (2, N = 385) = 137.8, p < 0.0001. 27, 21 and 150 LN (15.3%, 56.3%, 84.8%) and 120, 13 and 57 LN (67.8%, 90.2%, 32.2%) were identified by reader 2 on the two reads; χ2 (2, N = 388) = 102.4, p < 0.0001.
Conclusions
Adding DWI to T2WI significantly improved identification of pelvic LN by radiation oncologists and can therefore be regarded as a useful LN contouring technique for RT planning in pelvic malignancies.



Similar content being viewed by others
References
Fischer-Valuck BW, Rao YJ, Michalski JM. Intensity-modulated radiotherapy for prostate cancer. Transl Androl Urol. 2018;7(3):297–307.
Taylor A, Rockall AG, Powell ME. An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol). 2007;19(7):542–50.
Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi81–8.
Dinniwell R, Chan P, Czarnota G, Haider MA, Jhaveri K, Jewett M, et al. Pelvic lymph node topography for radiotherapy treatment planning from ferumoxtran-10 contrast-enhanced magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;74(3):844–51.
Vilarino-Varela MJ, Taylor A, Rockall AG, Reznek RH, Powell ME. A verification study of proposed pelvic lymph node localisation guidelines using nanoparticle-enhanced magnetic resonance imaging. Radiother Oncol. 2008;89(2):192–6.
Harris VA, Staffurth J, Naismith O, Esmail A, Gulliford S, Khoo V, et al. Consensus guidelines and contouring atlas for pelvic node delineation in prostate and pelvic node intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(4):874–83.
Potter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner K, Nout R, et al. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60.
Onjukka E, Uzan J, Baker C, Howard L, Nahum A, Syndikus I. Twenty fraction prostate radiotherapy with intra-prostatic boost: results of a pilot study. Clin Oncol (R Coll Radiol). 2017;29(1):6–14.
Caglic I, Barrett T. Diffusion-weighted imaging (DWI) in lymph node staging for prostate cancer. Transl Androl Urol. 2018;7(5):814–23.
Thoeny HC, Barbieri S, Froehlich JM, Turkbey B, Choyke PL. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology. 2017;285(3):728–43.
Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: what you need to know. Clin Radiol. 2015;70(11):1165–76.
Mulhall J, Ahmed A, Parker M, Mohideen N. The hemodynamics of erectile dysfunction following external beam radiation for prostate cancer. J Sex Med. 2005;2(3):432–7.
Morris KA, Haboubi NY. Pelvic radiation therapy: between delight and disaster. World J Gastrointest Surg. 2015;7(11):279–88.
Maturen KE, Feng MU, Wasnik AP, Azar SF, Appelman HD, Francis IR, et al. Imaging effects of radiation therapy in the abdomen and pelvis: evaluating “innocent bystander” tissues. Radiographics. 2013;33(2):599–619.
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–25.
Thoeny HC, Froehlich JM, Triantafyllou M, Huesler J, Bains LJ, Vermathen P, et al. Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology. 2014;273(1):125–35.
von Below C, Daouacher G, Wassberg C, Grzegorek R, Gestblom C, Sorensen J, et al. Validation of 3 T MRI including diffusion-weighted imaging for nodal staging of newly diagnosed intermediate- and high-risk prostate cancer. Clin Radiol. 2016;71(4):328–34.
McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology. 2010;254(1):31–46.
Ramirez M, Ingrand P, Richer JP, Herpe G, Vesselle G, Boucebci S, et al. What is the pelvic lymph node normal size? Determination from normal MRI examinations. Surg Radiol Anat. 2016;38(4):425–31.
Segedin B, Petric P. Uncertainties in target volume delineation in radiotherapy—are they relevant and what can we do about them? Radiol Oncol. 2016;50(3):254–62.
Vinod SK, Jameson MG, Min M, Holloway LC. Uncertainties in volume delineation in radiation oncology: a systematic review and recommendations for future studies. Radiother Oncol. 2016;121(2):169–79.
Hansen NL, Koo BC, Warren AY, Kastner C, Barrett T. Sub-differentiating equivocal PI-RADS-3 lesions in multiparametric magnetic resonance imaging of the prostate to improve cancer detection. Eur J Radiol. 2017;95:307–13.
Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol. 2017;90:174–80.
Gaffney DK, King B, Viswanathan AN, Barkati M, Beriwal S, Eifel P, et al. Consensus recommendations for radiation therapy contouring and treatment of vulvar carcinoma. Int J Radiat Oncol Biol Phys. 2016;95(4):1191–200.
Greenwalt JC, Amdur RJ, Morris CG, Morgan LS, Castagno J, Markham MJ, et al. Outcomes of definitive radiation therapy for primary vaginal carcinoma. Am J Clin Oncol. 2015;38(6):583–7.
Wilder RB, Buyyounouski MK, Efstathiou JA, Beard CJ. Radiotherapy treatment planning for testicular seminoma. Int J Radiat Oncol Biol Phys. 2012;83(4):e445–52.
Harrington K, Hall E, Hawkins M, Henry A, MacKay R, Maughan T, et al. Introducing the cancer research uk advanced radiotherapy technologies network (ART-NET). Clin Oncol (R Coll Radiol). 2017;29(11):707–10.
Acknowledgements
Author T. Barrett acknowledges support from Cancer Research UK, National Institute of Health Research Cambridge Biomedical Research Centre, Cancer Research UK and the Engineering and Physical Sciences Research Council Imaging Centre in Cambridge and Manchester and the Cambridge Experimental Cancer Medicine Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Cambridge University Hospitals Research Ethics Committee approved the study (CUH/03/018).
Informed consent
The study was approved by the institutional review board with the need for informed consent for data analysis waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sushentsev, N., Martin, H., Rimmer, Y. et al. Added value of diffusion-weighted MRI for nodal radiotherapy planning in pelvic malignancies. Clin Transl Oncol 21, 1383–1389 (2019). https://doi.org/10.1007/s12094-019-02068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02068-0