Abstract
Purpose
We report the response rate in children older than 18 months with stage 4 Neuroblastoma, using a modified dose-intensive, response-adaptive, induction mN7 protocol.
Methods
From 2005 to 2012, 24 patients were treated with the mN7 protocol. Phase 1 included five MSKCC N7 cycles and surgery and two high-dose cyclophosphamide-topotecan (HD-CT) cycles for those who did not achieve complete remission (CR) and negative bone marrow (BM) minimal residual disease (MRD) status (CR+MRD-). Phase 2 consisted of myeloablative doses of topotecan, thiotepa and carboplatin plus hyperfractionated RT. Phase 3 included isotretinoin and 3F8 immunotherapy plus GM-CSF. BM MRD was monitored using GD2 synthase, PHOX2B and cyclin D1 mRNAs.
Results
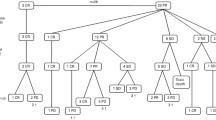
After 3 cycles, all patients showed BM complete histological clearance and 6 (25 %) were MRD-. Twenty of 21 s-look surgeries achieved macroscopic complete resection. After 5 cycles and surgery, 123I-MIBG scan was negative in 15 (62.5 %) cases, BM disease by histology was negative in 23 (96 %) and 10 (42 %) patients were MRD-. Twelve (50 %) pts were in CR, 2 in very good partial response (VGPR), 9 partial response (PR) and one had progressive disease. With 2 HD-CT extra cycles, 17 (71 %) pts achieved CR+MRD- status moving to phase 2. Overall and event-free survival at 3 years for the 17 patients who achieved CR+MRD- is 65 and 53 %, respectively, median follow-up 47 months. Seven (29 %) patients never achieved CR+MRD-. Univariate Cox regression analysis shows CR+MRD- status after mN7 induction as the only statistically significant prognostic factor to predict overall survival.
Conclusions
mN7 induction regimen produced a CR+MRD- rate of 71 %. CR+MRD- status following induction was the only predictive marker of long-term survival.

Similar content being viewed by others
References
Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11.
Kushner BH, Cohn SL, Matthay KK. Treatment of neuroblastoma. In: Cheung NKV, Cohn SL, editors. Neuroblastoma. Heidelberg: Springer-Verlag; 2005. p. 173–92.
Heller G, Cheung NK. Dose-intensity analysis and randomized trials. J Clin Oncol. 1991;9:1715–6.
Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–56.
Kushner BH, LaQuaglia MP, Bonilla MA, Lindsley K, Rosenfield N, Yeh S, et al. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J Clin Oncol. 1994;12:2607–13.
Cheung NK, Kushner BH, LaQuaglia M, Kramer K, Gollamudi S, Heller G, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001;36:227–30.
Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung NK. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–92.
Valteau-Couanet D. Event-free survival of patients with high-risk neuroblastoma treated with an N6-like induction treatment. J Clin Oncol. 2005;23:6262–3.
Kohler JA, Ellershaw C, Machin D. Neuroblastoma Working Group of the Children’s Cancer and Leukaemia Group. Response to N7 induction chemotherapy in children more than one year of age diagnosed with metastatic neuroblastoma treated in UKCCSG centers. Pediatr Blood Cancer. 2007;49:234–9.
Kushner BH, Cheung NK. Induction for high-risk neuroblastoma. Pediatr Blood Cancer. 2007;49:221–3.
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13.
Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008.
London WB, Frantz CN, Campbell LA, Seeger RC, Brumback BA, Cohn SL, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecanalone in children with recurrent or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2010;28:3808–15.
Kushner BH, Kramer K, Cheung NKV. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocytemacrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–94.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Cheung IY, Cheung NK. Quantitation of marrow disease in neuroblastoma by real-time reverse transcription-PCR. Clin Cancer Res. 2001;7:1698–705.
Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Kleijn I, Dee R, Hooft L, et al. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. J Clin Oncol. 2008;26:5443–9.
Cheung IY, Feng Y, Vickers A, Gerald W, Cheung NK. Cyclin D1, a novel molecular marker of minimal residual disease, in metastatic neuroblastoma. J Mol Diagn. 2007;9:237–41.
Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Yalcin B, Dee R, van Noesel MM, et al. Detecting minimal residual disease in neuroblastoma: the superiority of a panel of real-time quantitative PCR markers. Clin Chem. 2009;55:1316–26.
Kushner BH, Kramer K, Modak S, Qin LX, Cheung NK. Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer. 2010;116:3054–60.
Kushner BH, Cheung NK, Kramer K, Dunkel IJ, Calleja E, Boulad F. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transpl. 2001;28:551–6.
De Preter K, Speleman F, Combaret V, Lunec J, Laureys G, Eussen BH, et al. Quantification of MYCN, DDX1, and NAG gene copy number in neuroblastoma using a real-time quantitative PCR assay. Mod Pathol. 2002;15:159–66.
Beiske K, Burchil SA, Cheung IY, Hiyama E, Seeger RC, Cohn SL, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100:1627–37.
Mora J, de Torres C, Parareda A, Torner F, Galván P, Rodríguez E, et al. Treatment of Ewing sarcoma family of tumors with a modified P6 protocol in children and adolescents. Pediatr Blood Cancer. 2011;57:69–75.
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, Delabesse E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe against cancer program. Leukemia. 2003;17(12):2474–86.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77.
Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, et al. A new 123I-MIBG whole body scan scoring method-application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31:256–61.
Stutterheim J, Zappeij-Kannegieter L, Versteeg R, Caron HN, van der Schoot CE, Tytgat GA. The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer. 2011;47:1193–202.
Matthay KK, Edeline V, Lumbroso J, Tanguy ML, Asselain B, Zucker JM, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–91.
Katzenstein HM, Cohn SL, Shore RM, Bardo DM, Haut PR, Olszewski M, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–15.
Ladenstein R, Valteau-Couanet D, Brock P, Yaniv I, Castel V, Laureys G, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC inductionin pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–24.
Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot induction regimen incorporating pharmaco kinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29:4351–7.
Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–65.
Berthold F, Hero B. Neuroblastoma: current drug therapy recommendations as part of the total treatment approach. Drugs. 2000;59:1261–77.
Acknowledgments
This is an original article and has not been previously presented or submitted for publication. Generous donations from Alicia Pueyo and NEN (Asociación de padres de Niños Enfermos de Neuroblastoma) Foundations.
Conflict of interest
The authors have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mora, J., Cruz, O., Lavarino, C. et al. Results of induction chemotherapy in children older than 18 months with stage-4 neuroblastoma treated with an adaptive-to-response modified N7 protocol (mN7). Clin Transl Oncol 17, 521–529 (2015). https://doi.org/10.1007/s12094-014-1273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1273-8