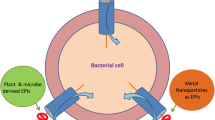
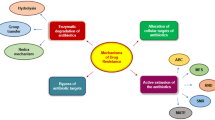
Abstract
Antimicrobial drugs have been noticed to have reduce activity effective due to upsurge witnessed in resistance of microbes. To deal with viewpoint of such a circumstance, we must seek ways to prevent it or atleast mitigate its effects in order to provide its activity against the microbes. Hence, novel antimicrobials are the one of the most promising solution for ending antimicrobial resistance. Furthermore, due to the less development of newer antimicrobials in recent years, the only way to combat microbial resistance are various synergistic approaches of exploring antimicrobial drug combinations. This combination's efficacy is due to a synergistic chemical that re-sensitizes the resistant microbial strain. It has been observed that classes of β-lactamases inhibitors, efflux pump inhibitors and membrane permeabilizers are of particular relevance, since they can break resistance to the most commonly used antimicrobials. This review explains the readers that how these synergistic combinations can help to reduce or eliminate the microbial resistance supported with clinical evidence.



Similar content being viewed by others
References
Ali J, Rafiq QA, Ratcliffe E (2018) Antimicrobial resistance mechanisms and potential synthetic treatments. Future Sci OA 4:FSO290. doi: https://doi.org/10.4155/fsoa-2017-0109
Reygaert C (2018) An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 4:482–501. https://doi.org/10.3934/microbiol.2018.3.482
Mulani MS, Kamble EE, Kumkar SN et al (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol . https://doi.org/10.3389/fmicb.2019.00539
Sg K, Adithan C, Harish B et al (2013) Antimicrobial resistance in India: a review. J Nat Sci Biol Med 4:286. https://doi.org/10.4103/0976-9668.116970
Organization WH (2014) Antimicrobial resistance: global report on surveillance. World Health Organization
Ganguly NK, Arora NK, Chandy SJ et al (2011) Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res 134:281–294
Chernov VM, Chernova OA, Mouzykantov AA et al (2019) Omics of antimicrobials and antimicrobial resistance. Expert Opin Drug Discov 14:455–468. https://doi.org/10.1080/17460441.2019.1588880
Kahn LH (2017) Antimicrobial resistance: a One Health perspective. Trans R Soc Trop Med Hyg 111:255–260. https://doi.org/10.1093/trstmh/trx050
Davies J, Davies D (2010) Origins and Evolution of Antibiotic Resistance. Microbiol Mol Biol Rev 74:417–433. https://doi.org/10.1128/MMBR.00016-10
Dixit A, Kumar N, Kumar S, Trigun V (2019) Antimicrobial resistance: progress in the decade since emergence of new Delhi metallo-β-lactamase in India. Indian J Community Med 44:4–8. https://doi.org/10.4103/ijcm.IJCM_217_18
Laws M, Shaaban A, Rahman KM (2019) Antibiotic resistance breakers: current approaches and future directions. FEMS Microbiol Rev 43:490–516. https://doi.org/10.1093/femsre/fuz014
Tyers M, Wright GD (2019) Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol 17:141–155. https://doi.org/10.1038/s41579-018-0141-x
Khilnani GC, Zirpe K, Hadda V et al (2019) Guidelines for antibiotic prescription in intensive care unit. Indian J Crit Care Med 23:S1–S63. https://doi.org/10.5005/jp-journals-10071-23101
Coates ARM, Hu Y, Holt J, Yeh P (2020) Antibiotic combination therapy against resistant bacterial infections: synergy, rejuvenation and resistance reduction. Expert Rev Anti Infect Ther 18:5–15. https://doi.org/10.1080/14787210.2020.1705155
Tamma PD, Cosgrove SE, Maragakis LL (2012) Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 25:450–470. https://doi.org/10.1128/CMR.05041-11
Cheesman M, Ilanko A, Blonk B, Cock I (2017) Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn Rev 11:57. https://doi.org/10.4103/phrev.phrev_21_17
Tooke CL, Hinchliffe P, Bragginton EC et al (2019) β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J Mol Biol 431:3472–3500. https://doi.org/10.1016/j.jmb.2019.04.002
Kumarasamy KK, Toleman MA, Walsh TR et al (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. https://doi.org/10.1016/S1473-3099(10)70143-2
González-Bello C, Rodríguez D, Pernas M et al (2020) β-Lactamase inhibitors to restore the efficacy of antibiotics against superbugs. J Med Chem 63:1859–1881. https://doi.org/10.1021/acs.jmedchem.9b01279
Watkins RR, Papp-Wallace KM, Drawz SM, Bonomo RA (2013) Novel β-lactamase inhibitors: a therapeutic hope against the scourge of multidrug resistance. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00392
Papp-Wallace KM, Bonomo RA (2016) New β-Lactamase inhibitors in the clinic. Infect Dis Clin North Am 30:441–464. https://doi.org/10.1016/j.idc.2016.02.007
Drawz SM, Bonomo RA (2010) Three decades of β-lactamase inhibitors. Clin Microbiol Rev 23:160–201. https://doi.org/10.1128/CMR.00037-09
Finlay J (2003) A review of the antimicrobial activity of clavulanate. J Antimicrob Chemother 52:18–23. https://doi.org/10.1093/jac/dkg286
White AR (2004) Augmentin(R) (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent. J Antimicrob Chemother 53:3i–20. https://doi.org/10.1093/jac/dkh050
Lai C-C, Chen C-C, Lu Y-C et al (2018) Appropriate composites of cefoperazone–sulbactam against multidrug-resistant organisms. Infect Drug Resist 11:1441–1445. https://doi.org/10.2147/IDR.S175257
Drawz SM, Papp-Wallace KM, Bonomo RA (2014) New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother 58:1835–1846. https://doi.org/10.1128/AAC.00826-13
Farrell DJ, Sader HS, Flamm RK, Jones RN (2014) Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. https://doi.org/10.1016/j.ijantimicag.2014.01.032
Crandon J, Nicolau D (2015) In vitro activity of cefepime/AAI101 and comparators against cefepime non-susceptible enterobacteriaceae. Pathogens 4:620–625. https://doi.org/10.3390/pathogens4030620
Crandon JL, Nicolau DP (2015) In vivo activities of simulated human doses of cefepime and cefepime-AAI101 against multidrug-resistant Gram-negative Enterobacteriaceae. Antimicrob Agents Chemother 59:2688–2694. https://doi.org/10.1128/AAC.00033-15
Sharma R, Park TE, Moy S (2016) Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination for the treatment of resistant gram-negative organisms. Clin Ther 38:431–444. https://doi.org/10.1016/j.clinthera.2016.01.018
Castanheira M, Mills JC, Costello SE et al (2015) ceftazidime-avibactam activity tested against enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother 59:3509–3517. https://doi.org/10.1128/AAC.00163-15
Vaara M (2019) Polymyxins and their potential next generation as therapeutic antibiotics. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01689
Schmid A, Wolfensberger A, Nemeth J et al (2019) Monotherapy versus combination therapy for multidrug-resistant gram-negative infections: systematic review and meta-analysis. Sci Rep 9:15290. https://doi.org/10.1038/s41598-019-51711-x
Vaara M (2019) Polymyxin derivatives that sensitize gram-negative bacteria to other antibiotics. Molecules 24:249. https://doi.org/10.3390/molecules24020249
Lin L, Nonejuie P, Munguia J et al (2015) Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. EBioMedicine 2:690–698. https://doi.org/10.1016/j.ebiom.2015.05.021
Sertcelik A, Baran I, Akinci E et al (2020) Synergistic activities of colistin combinations with meropenem, sulbactam, minocycline, disodium fosfomycin, or vancomycin against different clones of carbapenem-resistant acinetobacter baumannii strains. Microb Drug Resist 26:429–433. https://doi.org/10.1089/mdr.2019.0088
Vaara M (1992) Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395–411. https://doi.org/10.1128/mr.56.3.395-411.1992
Vaara M, Siikanen O, Apajalahti J et al (2010) A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob Agents Chemother 54:3341–3346. https://doi.org/10.1128/AAC.01439-09
Corbett D, Wise A, Langley T et al (2017) Potentiation of antibiotic activity by a novel cationic peptide: potency and spectrum of activity of SPR741. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00200-17
P Tegos, M Haynes, J Jacob Strouse et al (2011) Microbial Efflux Pump Inhibition: Tactics and Strategies. Curr Pharm Des 17:1291–1302. doi: https://doi.org/10.2174/138161211795703726
Sharma A, Gupta V, Pathania R (2019) Efflux pump inhibitors for bacterial pathogens: from bench to bedside. Indian J Med Res 149:129. https://doi.org/10.4103/ijmr.IJMR_2079_17
Willers C, Wentzel JF, du Plessis LH et al (2017) Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: the role of efflux inhibitors. Expert Opin Ther Targets 21:23–36. https://doi.org/10.1080/14728222.2017.1265105
Zhao Y-J, Liu W-D, Shen Y-N et al (2019) The efflux pump inhibitor tetrandrine exhibits synergism with fluconazole or voriconazole against Candida parapsilosis. Mol Biol Rep 46:5867–5874. https://doi.org/10.1007/s11033-019-05020-1
Li S-X, Song Y-J, Jiang L et al (2017) Synergistic effects of tetrandrine with posaconazole against aspergillus fumigatus. Microb Drug Resist 23:674–681. https://doi.org/10.1089/mdr.2016.0217
Tariq A, Sana M, Shaheen A, et al (2019) Restraining the multidrug efflux transporter STY4874 of Salmonella Typhi by reserpine and plant extracts. Lett Appl Microbiol lam. 13196. doi: https://doi.org/10.1111/lam.13196
Kumar A, Khan IA, Koul S et al (2008) Novel structural analogues of piperine as inhibitors of the NorA efflux pump of Staphylococcus aureus. J Antimicrob Chemother 61:1270–1276. https://doi.org/10.1093/jac/dkn088
Sharma S, Kumar M, Sharma S et al (2010) Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother 65:1694–1701. https://doi.org/10.1093/jac/dkq186
Stermitz FR, Lorenz P, Tawara JN et al (2000) Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci 97:1433–1437. https://doi.org/10.1073/pnas.030540597
Stapleton PD, Shah S, Anderson JC et al (2004) Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents 23:462–467. https://doi.org/10.1016/j.ijantimicag.2003.09.027
Kanagaratnam R, Sheikh R, Alharbi F, Kwon DH (2017) An efflux pump (MexAB-OprM) of Pseudomonas aeruginosa is associated with antibacterial activity of Epigallocatechin-3-gallate (EGCG). Phytomedicine 36:194–200. https://doi.org/10.1016/j.phymed.2017.10.010
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare no conflict of interest.
Ethical approval
Not applicable since it is a review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mhapankar, N., Siddique, A., Doshi, G. et al. Deciphering the Role of β-Lactamase Inhibitors, Membrane Permeabilizers and Efflux Pump Inhibitors as Emerging Targets in Antibiotic Resistance. Indian J Microbiol 62, 524–530 (2022). https://doi.org/10.1007/s12088-022-01045-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-022-01045-6