Abstract
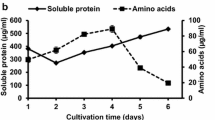
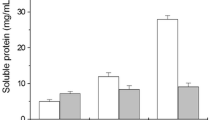
Keratinous waste is the bulk by-product of various livestock industries. Slow natural degradation of keratin and less efficient chemical hydrolysis imposes challenge for the search of alternative recycling methods. Keratin degrading microbes hydrolyse keratin to soluble peptides and amino acids. Bacillus aerius NSMk2 showed great potential for hydrolysis of chicken feather waste. Bacillus aerius NSMk2 cells grown in phosphate buffer supplemented with chicken feathers showed high disulfide reductase activity and release of sulfhydryl groups. The release of proteins and amino acids were statistically optimized at varied pH (4.0–11.0), temperature (30.0–45.0 °C) and agitation (100–250 rpm), and maximum release was recorded at pH 7.5, temperature 37 °C and shaking (175 rpm). FTIR and SEM showed sulfitolysis and extensive keratinolysis of feathers resulting in complete hydrolysis of white chicken feathers after 84 h. MALDI-TOF mass spectrometry confirmed the release of low molecular weight peptides in the range of 399 to 3289.4 m/z. The present study demonstrates management of otherwise hard-to-degrade keratinous waste and simultaneous nutritional enhancement of waste chicken feathers to value-added hydrolysate that can be used in livestock feed formulations or biofertilizer in agro-industry.


Similar content being viewed by others
References
Gupta S, Singh R (2014) Hydrolyzing proficiency of keratinases in feather degradation. Indian J Microbiol 54:466–470. https://doi.org/10.1007/s12088-014-0477-5
Gupta R, Rajput R, Sharma R, Gupta N (2013) Biotechnological applications and prospective market of microbial keratinases. Appl Microbiol Biotechnol 97:9931–9940. https://doi.org/10.3109/07388551.2012.685051
de Oliveira CC, de Souza AKS, de Castro RJS (2019) Bioconversion of chicken feather meal by Aspergillus niger: simultaneous enzyme production using a cost-effective feedstock under solid state fermentation. Indian J Microbiol 59:209–216. https://doi.org/10.1007/s12088-019-00792-3
Stiborova H, Branska B, Vesela T, Lovecka P, Stranska M, Hajslova J, Jiru M, Patakova P, Demnerova K (2016) Transformation of raw feather waste into digestible peptides and amino acids. J Chem Technol Biotechnol 91:1629–1637. https://doi.org/10.1002/jctb.4912
Go TH, Cho KS, Lee SM, Lee OM, Son HJ (2015) Simultaneous production of antifungal and keratinolytic activities by feather-degrading Bacillus subtilis S8. Indian J Microbiol 55:66–73. https://doi.org/10.1007/s12088-014-0502-8
Bhari R, Kaur M, Singh RS, Pandey A (2018) Bioconversion of chicken feathers by Bacillus aerius NSMk2: a potential approach in poultry waste management. Bioresour Technol Report 3:224–230. https://doi.org/10.1016/j.biteb.2018.07.015
Bhari R, Kaur M, Singh RS (2019) Thermostable and halotolerant keratinase from Bacillus aerius NSMk2 with remarkable dehairing and laundary applications. J Basic Microbiol 59:555–568. https://doi.org/10.1002/jobm.201900001
Brown DM, Upcroft JA, Upcroft P (1996) A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol Biochem Parasitol 8:211–220. https://doi.org/10.1016/S0166-6851(96)02776-4
Kunert J, Stransky Z (1988) Thiosulfate production from cystine by the keratinolytic prokaryote Streptomyces fradiae. Arch Microbiol 150:600–601
Bhushan B (2003) Adhesion and stiction: mechanisms, measurement techniques, and methods for reduction. J Vac Sci Technol B 21:2262–2296. https://doi.org/10.1116/1.1627336
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Lowry OH, Rosebrough N, Farr A, Randall J (1951) Protein estimation with Folin-phenol reagent. J Biol Chem 193:265–275
Moore S, Kunkel HG, Cole RD, Spackman DH, Stein WH (1957) Observations on the amino acid composition of human hemoglobins. Int J Microbiol 24:640–642
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using pre-column derivatization. J Chromatogr B Biomed Sci Appl 336:93–104. https://doi.org/10.1016/S0378-4347,85133-6
AOAC (2012) Official methods of analysis of the AOAC. Official J Eur Commun 4:18–19
Cai S, Singh BR (1999) Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys Chem 80:7–20. https://doi.org/10.1016/S0301-4622(99)00060-5
Gurav RG, Tang J, Jadhav JP (2016) Sulfitolytic and keratinolytic potential of Chryseobacterium sp. RBT revealed hydrolysis of melanin containing feathers. 3 Biotech 6:145. https://doi.org/10.1007/s13205-016-0464-0
Ramnani P, Singh R, Gupta R (2005) Keratinolytic potential of Bacillus licheniformis RG1: structural and biochemical mechanism of feather degradation. Can J Microbiol 51:191–196. https://doi.org/10.1139/w04-123
Wang B, Yang W, Mckittrick J, Meyers MA (2016) Keratin: structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog Mater Sci 76:229–318. https://doi.org/10.1016/j.pmatsci.2015.06.001
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39:549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x
Colla G, Nardi S, Cardarelli M, Ertani A, Lucini L, Canaguier R, Rouphael Y (2015) Protein hydrolysates as biostimulants in horticulture. Sci Hortic-Amsterdam 196:28–38. https://doi.org/10.1016/j.scienta.2015.08.037
Tiwary E, Gupta R (2012) Rapid conversion of chicken feather to feather meal using dimeric keratinase from Bacillus licheniformis ER-15. J Bioprocess Biotech 2:1–5. https://doi.org/10.4172/2155-9821.1000123
Paul T, Das A, Mandal A, Halder SK, Das Mohapatra PK, Pati BR, Mondal KC (2013) Valorization of chicken feather waste for concomitant production of keratinase, oligopeptides and essential amino acids under submerged fermentation by Paenibacillus woosongensis TKB2. Waste Biomass Valor 5:575–584. https://doi.org/10.1007/s12649-013-9267-2
Cortezi M, Cilli CM, Contiero J (2008) Bacillus amyloliquefaciens: a new keratinolytic feather-degrading bacteria. Curr Trends Biotechnol Pharm 2:170–177
He Z, Sun R, Tang Z et al (2018) Biodegradation of feather waste keratin by the keratin-degrading strain Bacillus subtilis 8. J Microbiol Biotechnol 28:314–322. https://doi.org/10.4014/jmb.1708.08077
Acknowledgments
The financial assistance received from Science and Engineering Research Board (SERB), Government of India, New Delhi in the form a major research project (ECR/2015/000156) is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhari, R., Kaur, M. & Singh, R.S. Nutritional Enhancement of Chicken Feather Waste by Bacillus aerius NSMk2. Indian J Microbiol 60, 518–525 (2020). https://doi.org/10.1007/s12088-020-00897-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-020-00897-0