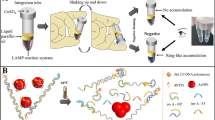
Abstract
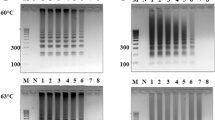
A limit of detection of 200 CFU/mL of Salmonella typhi spiked in various sample matrices were achieved in 30 min. The sample matrices were raw/unprocessed milk, commercially available milk, juice from packed bottles, fresh juice from carts, potable water, turbid water and calf serum. The complete protocol comprised of three steps: (a) cell lysis (b) nucleic acid amplification and (c) an in situ optical detection. The cell lysis was carried out using a simple heating based protocol, while the loop-mediated isothermal amplification of DNA was carried out by an in-house designed and fabricated system. The developed system consists of an aluminum block fitted with two cartridge heaters along with a thermocouple. The system was coupled to a light source and spectrometer for a simultaneous in situ detection. Primers specific for STY2879 gene were used to amplify the nucleic acid sequence, isolated from S. typhi cells. The protocol involves 15 min of cell lysis and DNA isolation followed by 15 min for isothermal amplification and simultaneous detection. No cross-reactivity of the primers were observed at 106 CFU/mL of Escherichia coli, Vibrio cholerae, Salmonella typhimurium, Salmonella paratyphi A, Pseudomonas aeruginosa, Bacillus cereus, Lysteria monocytogenes, Clostridium botulinum, Staphylococcus aureus and Salmonella havana. In addition, the system was able to detect S. typhi of 200 CFU/mL in a concoction of 106 CFU/mL of E. coli, 106 CFU/mL of V. cholerae, and 106 CFU/mL of hepatocyte-derived cellular carcinoma HUH7 cells. The proposed rapid diagnostic system shows a promising future in the field of food and medical diagnostics.






Similar content being viewed by others
References
Kalia VC, Kumar P (2015) Genome wide search for biomarkers to diagnose Yersinia infections. Indian J Microbiol 55:366–374
Ranjbar R, Mortazavi SM, Mehrabi Tavana A, Sarshar M, Najafi A, Zanjani RS (2017) Simultaneous molecular detection of Salmonella enterica Serovars Typhi, Enteritidis, Infantis, and Typhimurium, Iran. J Public Health 46:103–111
Seo JH, Park BH, Oh SJ, Choi G, Kim DH, Lee EY, Seo TS (2017) Development of a high-throughput centrifugal loop-mediated isothermal amplification microdevice for multiplex foodborne pathogenic bacteria detection. Sensors Actuators B Chem 246:146–153. https://doi.org/10.1016/j.snb.2017.02.051
Wachiralurpan S, Sriyapai T, Areekit S, Kaewphinit T, Sriyapai P, Santiwatanakul S, Chansiri K (2017) Development of a rapid screening test for Listeria monocytogenes in raw chicken meat using loop-mediated isothermal amplification (LAMP) and lateral flow dipstick (LFD). Food Anal Methods 10:3763–3772. https://doi.org/10.1007/s12161-017-0949-4
Karwa M, Currie B, Kvetan V (2005) Bioterrorism: preparing for the impossible or the improbable. Crit Care Med 33:S75–95
D’Agostino M, Robles S, Hansen F, Ntafis V, Ikonomopoulos J, Kokkinos P, Alvarez-Ordonez A, Jordan K, Delibato E, Kukier E et al (2016) Validation of a loop-mediated amplification/ISO 6579-based method for analysing soya meal for the presence of Salmonella enterica. Food Anal Methods 9:2979–2985
World Health Organization, Typhoid, World Heal. Organ (2015). http://www.who.int/immunization/diseases/typhoid/en/. Accessed Aug 5 2017
Fabiani L, Pucci E, Delibato E, Volpe G, Piermarini S, De Medici D, Capuano F, Palleschi G (2017) ELIME assay vs real-time PCR and conventional culture method for an effective detection of salmonella in fresh leafy green vegetables. Talanta 166:321–327. https://doi.org/10.1016/j.talanta.2017.01.071
Kumar M, Dahiya S, Sharma P, Sharma S, Singh TP, Kapil A, Kaur P (2015) Structure based in silico analysis of quinolone resistance in clinical isolates of Salmonella Typhi from India. PLoS ONE 10:e0126560
Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S (2013) Antimicrobial-resistant pathogens associated with healthcare- associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect Control Hosp Epidemiol 3:1–14. https://doi.org/10.1086/668770
Jain S, Chattopadhyay S, Jackeray R, Abid CKVZ, Kohli GS, Singh H (2012) Highly sensitive detection of Salmonella typhi using surface aminated polycarbonate membrane enhanced-ELISA. Biosens Bioelectron 31:37–43. https://doi.org/10.1016/j.bios.2011.09.031
Ahari H, Kakoolaki S, Anvar SAA (2017) Detection of Salmonella typhi using four developed kits of ELISA for cleaning in place purification. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1309-z
Preechakasedkit P, Pinwattana K, Dungchai W, Siangproh W (2012) Biosensors and bioelectronics development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens Bioelectron 31:562–566. https://doi.org/10.1016/j.bios.2011.10.031
Hsu H (2009) Development of enzyme linked, tissue blot and dot blot immunoassays for plant virus detection. In: Burns R (ed) Plant Pathology: technology and protocols. Humana Press, Totowa, pp 15–25. https://doi.org/10.1007/978-1-59745-062-1_2
Kalia VC, Kumar R, Kumar P, Koul S (2016) A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol 56:46–58
Kekre A, Bhushan A, Kumar P, Kalia VC (2015) Genome wide analysis for searching novel markers to rapidly identify Clostridium strains. Indian J Microbiol 55:250–257
Singh J, Sharma S, Nara S (2015) Nanogold based lateral flow assay for the detection of Salmonella typhi in environmental water samples. Anal Methods 7:9281–9288. https://doi.org/10.1039/C5AY02271A
Jain S, Chattopadhyay S, Jackeray R, Abid Z, Singh H (2016) Detection of Salmonella typhi utilizing bioconjugated fluorescent polymeric nanoparticles. J Nanoparticle Res 18:111. https://doi.org/10.1007/s11051-016-3414-1
Lazcka O, Del Campo FJ, Muñoz FX (2007) Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron 22:1205–1217. https://doi.org/10.1016/j.bios.2006.06.036
Notomi T, Mori Y, Tomita N, Kanda H (2015) Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 53:1–5. https://doi.org/10.1007/s12275-015-4656-9
Abdullah J, Saffie N, Sjasri FAR, Husin A, Abdul-Rahman Z, Ismail A, Aziah I, Mohamed M (2014) Rapid detection of Salmonella Typhi by loop-mediated isothermal amplification (LAMP) method. Braz J Microbiol 45:1385–1391
Kokkinos PA, Ziros PG, Bellou M, Vantarakis A (2014) Loop-mediated isothermal amplification (LAMP) for the detection of salmonella in food. Food Anal Methods 7:512–526. https://doi.org/10.1007/s12161-013-9748-8
Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knox GW, Ochoa-Corona FM, Paret ML (2017) A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods 240:78–84. https://doi.org/10.1016/j.jviromet.2016.11.014
Jiang Y, Zou S, Cao X (2017) A simple dendrimer-aptamer based microfluidic platform for E. coli O157:H7 detection and signal intensification by rolling circle amplification. Sensors Actuators B Chem 251:976–984. https://doi.org/10.1016/j.snb.2017.05.146
Hønsvall BK, Robertson LJ (2017) Real-time nucleic acid sequence-based amplification (NASBA) assay targeting MIC1 for detection of Cryptosporidium parvum and Cryptosporidium hominis oocysts. Exp Parasitol 172:61–67. https://doi.org/10.1016/j.exppara.2016.12.009
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63. https://doi.org/10.1093/nar/28.12.e63
Sayad AA, Ibrahim F, Uddin SM, Pei KX, Mohktar MS, Madou M, Thong KL (2016) A microfluidic lab-on-a-disc integrated loop mediated isothermal amplification for foodborne pathogen detection. Sensors Actuators B Chem 227:600–609. https://doi.org/10.1016/j.snb.2015.10.116
Fan F, Du P, Kan B, Yan M (2015) The development and evaluation of a loop-mediated isothermal amplification method for the rapid detection of Salmonella enterica serovar Typhi. PLoS ONE. https://doi.org/10.1371/journal.pone.0124507
Bozorgmehr A, Yazdanparast R, Hamidreza M (2016) Non-crosslinking gold nanoprobe-LAMP for simple, colorimetric, and specific detection of Salmonella typhi. J. Nanoparticle Res 18:351
Jin D-Z, Xu X-J, Chen S-H, Wen S-Y, Ma X-E, Zhang Z, Lin F, Wang S-Q (2007) Detection and identification of enterohemorrhagic Escherichia coli O157:H7 and Vibrio cholerae O139 using oligonucleotide microarray., Infect. Agent. Cancer 2:23. https://doi.org/10.1186/1750-9378-2-23
DL K, PH G (2007) Manual of clinical microbiology, 9th ed. American Society for Microbiology Press
Soni A, Jha SK (2015) A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens Bioelectron 67:763–768
Lin Y, Lu F, Tu Y, Ren Z (2004) Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett 4:191–195
Cai B, Huang L, Zhang H, Sun Z, Zhang Z, Zhang G-J (2015) Gold nanoparticles-decorated graphene field-effect transistor biosensor for femtomolar MicroRNA detection. Biosens Bioelectron 74:329–334
Acknowledgements
The authors would also like to acknowledge Professor Arti Kapil of All India Institute of Medical Sciences for valuable inputs.
Funding
The authors would like to acknowledge the funding agencies for their continued support: Department of Science and Technology (YSS/2014/000880, and IDP/MED/05/2014), Indo-German Science and Technology Centre (IGSTC/Call 2014/Sound4All/24/2015-16), Naval research board (NRB/4003/PG/359), BIRAC, Department of Biotechnology (BIRAC/BT/AIR0275/PACE-12/17).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
AK declares that she has no conflict of interest. RD declares that she has no conflict of interest. MRN declares that he has no conflict of interest. RE declares that he has no conflict of interest. DP declares that she has no conflict of interest. SJ declares that he has no conflict of interest. DK declares that he has no conflict of interest.
Ethical Approval
The study does not involve any human samples and there is no ethical clearance required for this work.
Rights and permissions
About this article
Cite this article
Kaur, A., Das, R., Nigam, M.R. et al. Rapid Detection Device for Salmonella typhi in Milk, Juice, Water and Calf Serum. Indian J Microbiol 58, 381–392 (2018). https://doi.org/10.1007/s12088-018-0730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-018-0730-4