Abstract
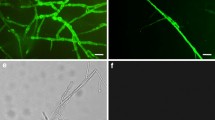
Physical contact between A. tumefaciens and the target plant cell walls is essential to transfer and integrate the transgene to introduce a novel trait. Chemotaxis response and attachment of Agrobacterium towards Vanda Kasem’s Delight (VKD) protocorm-like bodies (PLBs) were studied to analyse the interaction between Agrobacterium and PLB during the transformation event. The study shows that initially A. tumefaciens reversibly attached to PLB surface via polar and lateral mode of adherence followed by the irreversible attachment which involved the production of cellulosic fibril by A. tumefaciens. Cellulosic fibril allows formation of biofilm at the tip of trichome. Contrarily, attachment mutant Escherichia coli strain DH5α was significantly deficient in the attachment process. Spectrophotometric GUS assay showed the mean value of attachment by A. tumefaciens was 8.72 % compared to the negative control E. coli strain DH5α that produced 0.16 %. A. tumefaciens swarmed with sharper and brighter edge when severe wounding was applied to the PLBs producing the highest swarming ratio of 1.46 demonstrating the positive effect of the plant exudates on bacterial movement. The study shows that VKD’s PLBs are the suitable explants for Agrobacterium-mediated transformation since the bacteria expressed higher competency rate.


Similar content being viewed by others
References
Anderson DJ, Birch RG (2012) Minimal handling and super-binary vector facilitate efficient Agrobacterium-mediated transformation of sugarcane (Saccharum spp. hybrid). Trop Plant Biol 5:183–192. doi:10.1007/s12042-012-9101-1
Shantha SL, Padma Bhat SG, Sunil Kumar C, Ragavendra Rao B (2012) Genetic manipulation of tomato (Lycopersicon esculentum) using wga gene through Agrobacterium-mediated transformation. Kathmandu Univ J Sci Eng Technol 8:36–43. doi:10.3126/kuset.v8i1.6041
Nizam S, Verma S, Singh K, Aggarwal R, Srivastava KD, Verma PK (2012) High reliability transformation of the wheat pathogen Bipolaris sorokiniana using A. tumefaciens. J Microbiol Methods 88:386–392. doi:10.1016/J.MIMET.2012.01.004
Sreeramanan S, Vinod B, Sashi S, Xavier R (2008) Optimization of the transient gus A gene transfer of Phalaenopsis violacea orchid via Agrobacterium tumefaciens: an assessment of factors influencing the efficiency of gene transfer mechanisms. Adv Nat Appl Sci 2:77–88
Ng CY, Saleh NM (2011) In vitro propagation of Paphiopedilum orchid through formation of protocorm-like bodies. Plant Cell Tissue Org 105:193–202. doi:10.1007/s11240-010-9851-0
Vacin EF, Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613. doi:10.1086/335561
Perez Hernandez JB, Remy S, Sauco GV, Swennen R, Sagi L (1999) Chemotactic movement and attachment of A. tumefaciens to banana cells and tissues. J Plant Physiol 155:245–250 (0176-1617/99/155/245)
Wilson KJ, Hughes SG, Jefferson RA. The Escherichia coli gus operon: Induction and expression of the gus operon in E. coli and the occurrence and use of GUS in other bacteria (1992) In: Gallagher SR (ed) GUS protocols: Using the GUS gene as a reporter of gene expression. Academy Press Inc, San Diego, pp 7–22
Remans T, Schenk PM, Manners JM, Grof CPL, Elliot AR (1999) A protocol for the fluorometric quantification of mGFPS-ER and sGFP (S65T) in transgenic plants. Plant Mol Biol Rep 17:385–395. doi:10.1023/A:1007654318401
Shaw CH (1995) A. tumefaciens chemotaxis protocols. In: Gartland KMA, Davey MR (eds) Agrobacterium protocols: methods in molecular biology. Humana Press Inc, Totowa, NJ, pp 29–36. doi:10.1385/0-89603-302-3:29
Gelvin SB (2010) Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol 48:45–68. doi:10.1146/annurev-phyto-080508-081852
Fronzes R, Christie PJ, Waksman G (2009) The structural biology of type IV secretion systems. Nat Rev Microbiol 7:703–714. doi:10.1038/nrmicro2218
Aly KA, Krall L, Lottspeich F, Baron C (2008) The Type IV secretion system component VirB5 binds to the trans-zeatin biosynthetic enzyme Tzs and enables its translocation to the cell surface of Agrobacterium tumefaciens. J Bacteriol 190:1595–1604. doi:10.1128/JB.01718-07
Judd PK, Kumar RB, Das A (2005) Spatial location and requirements for the assembly of the A. tumefaciens type IV secretion apparatus. P Natl Acad Sci USA 102:11498–11503. doi:10.1073/pnas.0505290102
Tomlinson AD, Fuqua C (2009) Mechanisms and regulation of polar surface attachment in A. tumefaciens. Curr Opin Microbiol 12:708–714. doi:10.1016/j.mib.2009.09.014
Atmakuri K, Cascales E, Burton OT, Banta LM, Christie PJ (2007) Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions. EMBO J 26:2540–2551. doi:10.1038/sj.emboj.7601696
Aguilar J, Cameron TA, Zupan J, Zambryski P (2011) Membrane and core periplasmic A. tumefaciens virulence Type IV secretion system components localize to multiple sites around the bacterial perimeter during lateral attachment to plant cells. MBio 2:e00218–11. doi:10.1128/mBio.00218-11
Rodríguez-Navarro DN, Dardanelli MS, Ruíz-Saínz JE (2007) Attachment of bacteria to the roots of higher plants. FEMS Microbiol Lett 272:127–136. doi:10.1111/j.1574-6968.2007.00761.x
Amin MCIM, Abadi AG, Ahmad N, Katas H, Jamal JA (2012) Bacterial cellulose film coating as drug delivery system: physicochemical, thermal and drug release properties. Sains Malays 41:561–568
Marshall SH, Gómez FA, Ramírez R, Nilo L, Henríquez V (2012) Biofilm generation by Piscirickettsia salmonis under growth stress conditions: a putative in vivo survival/persistence strategy in marine environments. Res Microbiol 163:557–566. doi:10.1016/j.resmic.2012.08.002
Gelvin SB (2006) Agrobacterium virulence gene induction. In: Wang K (ed) Methods in molecular biology Agrobacterium protocols. Humana Press Inc, Totowa, pp 77–84 (ISBN 1-58829-536-2)
Gelvin SB (2009) Agrobacterium in the genomics age. Plant Physiol 150:1665–1676. doi:10.1104/pp.109.139873
Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL (2005) pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J Bacteriol. doi:10.1128/JB.187.1.304-319.2005
Logan SM (2006) Flagellar glycosylation: a new component of the motility repertoire. Microbiology 152:1249–1262. doi:10.1099/mic.0.28735-0
Acknowledgments
The authors gratefully acknowledge the financial support provided by Universiti Sains Malaysia (USM) through the Research University Grant 2012.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gnasekaran, P., Subramaniam, S. Mapping of the Interaction Between Agrobacterium tumefaciens and Vanda Kasem’s Delight Orchid Protocorm-Like Bodies. Indian J Microbiol 55, 285–291 (2015). https://doi.org/10.1007/s12088-015-0519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-015-0519-7