Abstract
We consider eco-evolutionary processes that include natural bounds on adaptive trait distributions. We implement ecological axioms, that a population grows if it is replete with resources, and doesn’t grow if it has none. These axioms produce natural bounds on the trait means that suggest that the assumption of gamma-distributed traits, where the trait variance is a function of the trait mean, is more appropriate than the usual assumption of normally distributed traits, where the trait variance is independent of the trait mean. We use a Lotka-Volterra model to simulate two plant populations, whose trait means evolve according to an evolutionary model, to simulate populations adapting during invasions. The results of our model simulations using gamma-distributed traits suggest that adapting populations may endure bottlenecks by increasing their fitness and recovering from near extinction to stably coexist. The inclusion of eco-evolutionary processes into ecosystem models generates K∗ theory which predicts the long-term states of populations that make it through evolutionary bottlenecks. Otherwise, the final states of populations that do not make it through bottlenecks may be predicted by the non-evolutionary R∗ theory.


Similar content being viewed by others
Change history
14 March 2019
The original version of this article unfortunately contains an incorrect panel (b) in Fig. 1 introduced during the production process.
References
Abrams PA (1991) The effects of interacting species on predator-prey coevolution. Theor Pop Biol 39:241–262
Abrams PA, Matsuda H, Harada Y (1993) Evolutionarily unstable fitness maxima and stable fitness minima of continuous traits. Evol Ecol 7:465–487
Abrams PA (1999) The adaptive dynamics of consumer choice. Am Nat 153:83–97
Abrams PA, Matsuda H (2004) Consequences of behavioral dynamics for the population dynamics of predator-prey systems with switching. Popul Ecol 46:13–25
Behrman KD, Kirkpatrick M (2011) Species range expansion by 749 beneficial mutations. J Evol Biol 24:665–675
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Cortez MH, Ellner SP (2010) Understanding rapid evolution in predator-prey interactions using the theory of fast-slow dynamical systems. Am Nat 176(5):E109–E127
Cortez MH (2016) How the magnitude of prey genetic variation alters predator-prey eco-evolutionary dynamics. Am Nat 188:329–341
Cortez MH, Patel S (2017) The effects of predator-evolution and genetic variation on predator-prey population-level dynamics. B Math Biol 79:1510–1538
Coutinho RM, Klauschies T, Gaedke U (2016) Bimodal trait distributions with large variances question the reliability of trait-based aggregate model. Theor Ecol 9:389–408
Cropp RA, Norbury J (2015) Population interactions in ecology: a rule-based approach to modeling ecosystems in a mass-conserving framework. SIAM Rev 57(3):437–465
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21(3):130–135
Falconer DS (1960) Introduction to quantitative genetics. Robert MacLeHose and Co
Fisher R (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Fox JW, Nelson WA, McCauley E (2010) Coexistence mechanisms and the paradox of the plankton: quantifying selection from noisy data. Ecol 91(6):1774–1786
Fussman GF, Ellner SP, Hairston NG Jr (2003) Evolution as a critical component of plankton dynamics. P R Soc London B Bio 270:1015–1022
Fussman GF, Loreau M, Abrams PA (2007) Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol 21(3):465–477
Gaedke U, Klauschies T (2017) Analysing the shape of observed trait distributions enables a data-based moment closure of aggregate models. L&O Methods 15:979–994
Gause G (1934) The struggle for existence. Williams & Wilkins
Grafen A (2015) Biological fitness and the fundamental theorem of natural selection. Am Nat 186(1):1–14
Hutchinson GE (1961) The paradox of the plankton. Am Nat 95:137–145
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol S 39:321–342
Klauschies T, Coutinho RM, Gaedke U (2018) A beta distribution-based moment closure enhances the reliability of trait-based aggregate models for natural populations and communities. Ecol Model 381:46–77
Kremer CT, Klausmeier CA (2013) Coexistence in a variable environment: eco-evolutionary perspectives. J Theor Biol 339:14–25
Lande R (1976) Natural selection and random genetic drift in phenotype evolution. Evolution 30:314–334
Lande R (1982) A quantitative genetic theory of life history evolution. Ecol 63:607–615
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17(8):386–391
Loreau M (2010) From populations to ecosystems. Princeton University Press, Princeton
Lotka AJ (1925) Elements of physical biology. Wilkins and Kilkins
Merico A, Bruggeman B, Wirtz K (2009) A trait-based approach for downscaling complexity in plankton ecosystem models. Ecol Model 220:3001–3010
Osmond MM, Otto SP, Klausmeier CA (2017) When predators help prey adapt and persist in a changing environment. Am Nat 190: 83–98
Pastor J (2017) Ecosystem ecology and evolutionary biology, a new frontier for experiments and models. Ecosystems 20:245–252
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22(9):454–464
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sasaki A, Dieckmann U (2011) Oligomorphic dynamics for analyzing the quantitative genetics of adaptive speciation. J Math Biol 63:601–635
Schoener TW (2011) The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331:426–429
Tilman D (1977) Resource competition between planktonic algae: an experimental and theoretical approach. Ecol 58:338–348
Tilman D (1981) Tests of resource competition theory using four species of lake michigan algae. Ecol 62:802–815
Tilman D (2007) Interspecific competition and multispecies coexistence. In: May RM, McLean A (eds) Theoretical ecology. Oxford University Press, London, p 257
Vasseur DA, Amarasekare P, Rudolf VHW, Levine JM (2011) Eco-evolutionary dynamics enable coexistence via neighbor-dependent selection. Am Nat 178(5):E96–E109
Vitousek PM, Matson PA (2012) Nutrient cycling and biogeochemistry. In: Levin S (ed) The princeton guide to ecology. Princeton University Press, Princeton, pp 330–339
Volterra V (1926) Variations and fluctuations of the numbers of individuals in animal species living together. In: Chapman RN (ed) 1931 animal ecology. McGraw Hill, New York
Yoshida T, Hairston NG Jr, Ellner SP (2004) Evolutionary trade-off between defence against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc R Soc Lond B 271:1947–1953
Acknowledgements
The authors thank two anonymous reviewers for their thoughtful and helpful comments that considerably improved this manuscript.
Funding
RC thanks the Mathematical Institute of the University of Oxford for providing funding to support this research.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the conception and design of the study, and the writing of the manuscript. RC did the numerical analysis and produced the figures and data.
Corresponding author
Appendix: Traits with bounded distributions
Appendix: Traits with bounded distributions
The bounds upon the values that some traits may take, that naturally arise from the ecological axiom that all viable populations require resources to grow (i.e. that \(f_{i}|_{R_{i}= 1}>0>f_{i}|_{R_{i}= 0}\)), suggest that some phenotypes have gamma distributions as they are constrained to be positive, negative, or greater or lesser than other parameters. Such constraints arise naturally in LVCN systems, and are fundamental to the system being ecologically valid. Here, where Eq. 1 represents competition between two populations, one incumbent and one potential invader, the parameter relations (2) are fundamental.
We follow the derivation of Lande (1976) and consider the evolution of phenotypes based on an equation provided by Falconer (1960) that describes the deterministic change in the average value of a phenotypic character \(\bar {z}\) in response to selection:
where \(\bar {z}(t)\) is the mean value of the character in generation t before selection, \(\bar {z}_{w}(t)\) is the mean value of the character after selection, but before reproduction, and h2 is the realised heritability of the character. We denote the distribution of phenotypes z in the generation t before selection as p(z, t), and then the average phenotype before selection is:
the mean fitness of individuals in the population is:
and the average phenotype after selection is:
Then assuming that the phenotype has a γ-distribution:
where we have used \(\bar {z}(t)=\alpha / \beta \) to eliminate β. This gives the change in mean fitness of the population (\(\bar {W}\)) with respect to a change in the average phenotype (\(\bar {z}(t)\)) as:
Then combining (15) with (10) gives:
Note that \(\bar {z}(t)/\beta \) is equivalent to the variance of the distribution, and represents the change in variance of the distribution as the mean approaches the lower bound (zero in the general case). As parameters in LVCN models may have lower bounds other than zero, for example aij in Eq. 1, we allow for a non-zero lower bound zL in Eq. 16 and assume continuous trait variation:
Equation 17 ensures that phenotypes with bounded distributions are not able to exceed their bounds during simulations.
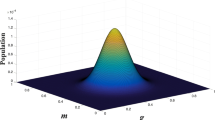
The key attribute of traits that have gamma distributions for eco-evolutionary modelling is that the mean value of the trait is bounded and the variance is related to the mean trait value, so that as the mean of the trait approaches its bound the variance of the distribution of the trait reduces.
Rights and permissions
About this article
Cite this article
Cropp, R., Norbury, J. An eco-evolutionary system with naturally bounded traits. Theor Ecol 12, 401–412 (2019). https://doi.org/10.1007/s12080-019-0407-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-019-0407-6