Abstract
The current pandemic caused by the SARS-CoV-2 has claimed over a half a million lives within a very short span of time. A therapeutic drug which could prevent the entry and propagation of the virus is the need of the hour. Several lines of evidence collected from experimental studies older than three decades have pointed out the fact that inhibiting calcium entry into cells can affect vital steps in the lifecycle of viruses. Hence, calcium channel blockers may be considered as an effective measure in the containment of the viruses. This commentary throws light two scientific papers although with divergent facts converging at a point by suggesting a promising treatment option for CoVID-19 (Fang et al. Lancet Respir Med 8:e21, 2020; Straus et al. J Virol 94:e00426, 2020).
Similar content being viewed by others
The association of CoVID-19 infection with co-morbid conditions such as diabetes and hypertension is considered to be a fatal combination in elderly population. The process of host cell invasion is initiated by the recognition of ACE2 receptor (Angiotensin converting enzyme 2) expressed in the epithelial cells of major organs such as lung, kidney and intestine (Fang et al. 2020; Paramasivam et al. 2020). ACE inhibitors, angiotensin II type-I receptor blockers (ARBs), thiazolidinediones and ibuprofen have been reported to increase the expression of ACE2, thereby increasing the risk of infection (Kai and Kai 2020; Li et al. 2020). An alternative strategy could be to use other forms of anti-hypertensive drugs with a special emphasis on calcium channel blockers. Why calcium channel blockers? Viruses greatly rely on host’s calcium homeostasis to modulate signal transduction pathways in its favour. As convincing reports on calcium channel blockers mediated increase in ACE2 expression has not been documented so far, these drugs can be a safe alternative for treating CoVID patients with hypertension.
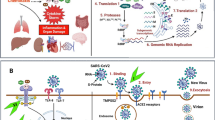
Host cells are found to modulate calcium signalling in response to viral infection and the viruses in-turn harness this environment for their own survival and propagation. Elevation in cytosolic calcium concentration and influx of calcium into mitochondria supports viral replication. Regulation of calcium in endoplasmic reticulum (ER) and Golgi apparatus, further aids in the inhibition of host protein trafficking and viral protein maturation. Various calcium channels promote the lifecycle of virus in the cell by supporting entry, replication, assembly and release of virions. Elevated calcium influx via store-operated calcium channels (SOC) contribute to the establishment of infection due to rotavirus. The transient receptor potential channel (TRP) is known to be associated with hypersensitivity induced by chemical or thermal stimuli. Infection of human bronchial epithelial cells by respiratory viruses including respiratory syncytial virus (RSV), measles virus (MV) and rhinovirus (RV) was found to increase the expression of TRP channels in human bronchial epithelial cells. The over-expression of TRP proteins provides a favourable environment for propagation of virus (Chen et al. 2019; Omar et al. 2017). Infections caused by influenza A virus was successfully contained by Verapamil, a drug to treat hypertension, atrial fibrillation and angina. Compounds blocking L-type calcium channels such as nimodipine, diltiazem etc., and a blocker of voltage gated calcium channel (VGCC) was found to inhibit Ebola virus in vivo. Hence targeting calcium channels might resolve issues pertaining to viral infections.
A recent study demonstrated that over a two-fold increase in intracellular Ca2+ enhanced the fusogenic ability of MERS-CoV. A similar study on SARS-CoV provided substantial evidence on the requirement of Ca2+ by the bipartite fusion platform of the viral peptide enabling fusion and entry into the host cells. Furthermore, the entry of virions through plasma membrane and endosomes were significantly reduced upon depletion of intra and extracellular calcium ions (Straus et al. 2020). More recent studies on Ebola virus have also reinforced the fact that Ca2+ ions enhanced their entry via interactions with negatively charged residues D522 and E540 of the fusion peptide (Nathan et al. 2020). Research by Chang and colleagues on the dysregulation of calcium homeostasis during Rota virus infection also validated the fact. A nonstructural glycoprotein (NSP4) localized on the ER acts as a viroporin or virus encoded ion channel to mediate efflux of Ca2+ ions from the ER. A sustained increase in cytosolic Ca2+ and decreased ER Ca2+ is sensed by stromal interaction molecule 1 which in turn assists the entry of Ca2+ through SOC (Chang-Graham et al. 2019).
A very recent report had re-proposed the use of verapamil as modulators of endosomal trafficking, by inhibition of both Ca2+ and K+ channels in Simian virus 40 (SV40). In addition, they proved that Merkel cell polyomavirus (MCPyV) and SV40 inevitably rely on two-pore Ca2+ channels (TPC) for capsid disassembly and nuclear transport which was blocked using tetrandrine, a TPC specific blocker (Dobson et al. 2020). Interestingly, in an attempt made to identify genetic variants associated with susceptibility to neuro-invasive disease caused by West Nile virus, CASNA1H (Calcium Voltage-Gated Channel Subunit Alpha1 H) achieved a genome-wide significance implying the fact that ion-channels could be as important as other immuno-modulatory proteins controlling host response to viral infections (Long et al. 2016). Taken together, investigations on common pathways employed by different viruses to create an intracellular Ca2+ storm potentiating the needs of the virus to propagate within the host cells would lead to identification of those prescription drugs as a magical cure for sudden pandemics. Extensive research on existing calcium channel blockers intended for use as anti-hypertensives might as well aid in repurposing these drugs into the anti-viral regimen.
References
Chang-Graham AL, Perry JL, Strtak AC, Ramachandran NK, Criglar JM, Philip AA, Patton JT, Estes MK, Hyser JM (2019) Rotavirus calcium dysregulation manifests as dynamic calcium signaling in the cytoplasm and endoplasmic reticulum. Sci Rep 9:10822
Chen X, Cao R, Zhong W (2019) Host calcium channels and pumps in viral infections. Cells 9(1):94
Dobson SJ, Mankouri J, Whitehouse A (2020) Identification of potassium and calcium channel inhibitors as modulators of polyomavirus endosomal trafficking. Antivir Res 7:104819
Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8:e21
Kai H, Kai M (2020) Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res 43(7):648–654
Li G, Hu R, Zhang X (2020) Antihypertensive treatment with ACEI/ARB of patients with COVID19 complicated by hypertension. Hypertens Res 43(6):588–590
Long D, Deng X, Singh P, Loeb M, Lauring AS, Seielstad M (2016) Identification of genetic variants associated with susceptibility to West Nile virus neuroinvasive disease. Genes Immun 7:298–304
Nathan L, Lai AL, Millet JK, Straus MR, Freed JH, Whittaker GR, Daniel S (2020) Calcium ions directly interact with the Ebola virus fusion peptide to promote structure-function changes that enhance infection. ACS Infect Dis 6:250–260
Omar S, Clarke R, Abdullah H, Brady C, Corry J, Winter H, Touzelet O, Power UF, Lundy F, McGarvey LPA, Cosby SL (2017) Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One 12(12):e0171681
Paramasivam A, Vijayashree Priyadharsini J, Raghunandhakumar S, Elumalai P (2020) A novel COVID-19 and its effects on cardiovascular disease. Hypertens Res 43(7):729–730
Straus MR, Tang T, Al L, Flegel A, Bidon M, Freed JH, Daniel S, Whittaker GR (2020) Ca2+ ions promote fusion of Middle East respiratory syndrome coronavirus with host cells and increase infectivity. J Virol 94:e00426–e00420
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jayaseelan, V.P., Paramasivam, A. Repurposing calcium channel blockers as antiviral drugs. J. Cell Commun. Signal. 14, 467–468 (2020). https://doi.org/10.1007/s12079-020-00579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-020-00579-y