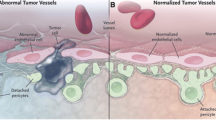
Abstract
Tumor neovascularization/tumor angiogenesis is a pathophysiological process in which new blood vessels are formed from existing blood vessels in the primary tumors to supply adequate oxygen and nutrition to cancer cells for their proliferation and metastatic growth to the distant organs. Therefore, controlling tumor angiogenesis is an attractive target for cancer therapy. Structural abnormalities of the vasculature (i.e., leakiness due to the abnormal lining of pericytes on the microvessels) are one of the critical features of tumor angiogenesis that sensitizes vascular cells to cytokines and helps circulating tumor cells to metastasize to distant organs. Our goal is to repurpose the drugs that may prevent tumor angiogenesis or normalize the vessels by repairing leakiness via recruiting pericytes or both. In this study, we tested whether aspirin (ASA), which could block primary tumor growth, regulates tumor angiogenesis. We investigated the effects of low (1 mM) and high (2.5 mM) doses of ASA (direct effect), and ASA-treated or untreated triple negative breast cancer (TNBC) cells’ conditioned media (indirect effect) on endothelial cell physiology. These include in vitro migration using modified Boyden chamber assay, in vitro capillary-like structure formation on Matrigel, interactions of pericytes-endothelial cells and cell permeability using in vitro endothelial permeability assay. We also examined the effect of ASA on various molecular factors associated with tumor angiogenesis. Finally, we found the outcome of ASA treatment on in vivo tumor angiogenesis. We found that ASA-treatment (direct or indirect) significantly blocks in vitro migration and capillary-like structure formation by endothelial cells. Besides, we found that ASA recruits pericytes from multipotent stem cells and helps in binding with endothelial cells, which is a hallmark of normalization of blood vessels, and decreases in vitro permeability through endothelial cell layer. The antiangiogenic effect of ASA was also documented in vivo assays. Mechanistically, ASA treatment blocks several angiogenic factors that are associated with tumor angiogenesis, and suggesting ASA blocks paracrine-autocrine signaling network between tumor cells and endothelial cells. Collectively, these studies implicate aspirin with proper dose may provide potential therapeutic for breast cancer via blocking as well as normalizing tumor angiogenesis.







Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ASA :
-
Aspirin
- VEGF :
-
Vascular Endothelial Growth Factor
- HUVEC :
-
Human Umbilical Vein Endothelial Cell
- CM :
-
Conditioned Media
References
Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Kato I, Koenig KL, Shore RE (2001) Aspirin and epithelial ovarian cancer. Prev Med 33:682–687
Ararat E, Sahin I, Altundag K (2011) Aspirin intake may prevent metastasis in patients with triple-negative breast cancer. Med Oncol 28:1308–1310
Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14:53–65
Azzi S, Hebda JK, Gavard J (2013) Vascular permeability and drug delivery in cancers. Front Oncol 3:211
Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM (2003) Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 163:1801–1815
Bardia A, Olson JE, Vachon CM, Lazovich DA, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR (2011) Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat 126:149–155
Bazzoni G, Dejana E (2004) Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84:869–901
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 7:452–464
Bielenberg DR, Zetter BR (2015) The contribution of angiogenesis to the process of metastasis. Cancer J 21:267–273
Bosetti C, Rosato V, Gallus S, Cuzick J, la Vecchia C (2012) Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol 23:1403–1415
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10:417–427
Claesson-Welsh L (2015) Vascular permeability--the essentials. Ups J Med Sci 120:135–143
Cooke VG, LeBleu VS, Keskin D et al (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21:66–81
Das A, Dhar K, Maity G, Sarkar S, Ghosh A, Haque I, Dhar G, Banerjee S, Banerjee SK (2017) Deficiency of CCN5/WISP-2-driven program in breast cancer promotes Cancer epithelial cells to mesenchymal stem cells and breast Cancer growth. Sci Rep 7:1220
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Dhar K, Dhar G, Majumder M, Haque I, Mehta S, van Veldhuizen PJ, Banerjee SK, Banerjee S (2010) Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer 9:209
Din FV, Dunlop MG, Stark LA (2004) Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br J Cancer 91:381–388
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29:15–18
German AE, Mammoto T, Jiang E, Ingber DE, Mammoto A (2014) Paxillin controls endothelial cell migration and tumor angiogenesis by altering neuropilin 2 expression. J Cell Sci 127:1672–1683
Giverso C, Ciarletta P (2016) Tumour angiogenesis as a chemo-mechanical surface instability. Sci Rep 6:22610
Huang Y, Lichtenberger LM, Taylor M, Bottsford-Miller JN, Haemmerle M, Wagner MJ, Lyons Y, Pradeep S, Hu W, Previs RA, Hansen JM, Fang D, Dorniak PL, Filant J, Dial EJ, Shen F, Hatakeyama H, Sood AK (2016) Antitumor and antiangiogenic effects of aspirin-PC in ovarian Cancer. Mol Cancer Ther 15:2894–2904
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Laitakari J, Nayha V, Stenback F (2004) Size, shape, structure, and direction of angiogenesis in laryngeal tumour development. J Clin Pathol 57:394–401
Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100:782–794
Liao D, Johnson RS (2007) Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 26:281–290
Longatto Filho A, Lopes JM, Schmitt FC (2010) Angiogenesis and breast cancer. J Oncol 2010:1–7
Luk K, Boatman S, Johnson KN et al (2012) Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy. J Oncol 2012:458385
Mahadevan V, Hart IR (1990) Metastasis and angiogenesis. Acta Oncol 29:97–103
Maity G, Mehta S, Haque I et al (2014) Pancreatic tumor cell secreted CCN1/Cyr61 promotes endothelial cell migration and aberrant neovascularization. Sci Rep 4:4995
Maity G, De A, Das A et al (2015) Aspirin blocks growth of breast tumor cells and tumor-initiating cells and induces reprogramming factors of mesenchymal to epithelial transition. Lab Investig 95:702–717
Martin TA, Jiang WG (2009) Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 1788:872–891
Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, Tsai YC, Williams EH, Lee DH, Stephens RM, Weissman AM, Ambs S (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 4:e4531
Mitra S, Wang X, Khaidakov M, Ding Z, Ayyadevera S, Hearnsberger E, Goyal T, Mehta JL (2012) Aspirin downregulates angiotensin type 1 receptor transcription implications in capillary formation from endothelial cells. J Cardiovasc Pharmacol 60:187–192
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2:213–219
Raza A, Franklin MJ, Dudek AZ (2010) Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol 85:593–598
Ribeiro AL, Okamoto OK (2015) Combined effects of pericytes in the tumor microenvironment. Stem Cells Int 2015:868475
Sarkar S, Ghosh A, Banerjee S, Maity G, Das A, Larson MA, Gupta V, Haque I, Tawfik O, Banerjee SK (2017) CCN5/WISP-2 restores ER- proportional, variant in normal and neoplastic breast cells and sensitizes triple negative breast cancer cells to tamoxifen. Oncogenesis 6:e340
Senger DR, Galli SJ, Dvorak AM et al (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Sennino B, Falcon BL, McCauley D, le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM (2007) Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res 67:7358–7367
Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS (2015) ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 208:821–838
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370
Xian X, Hakansson J, Stahlberg A et al (2006) Pericytes limit tumor cell metastasis. J Clin Invest 116:642–651
Zhang X, Wang Z, Wang Z et al (2013) Impact of acetylsalicylic acid on tumor angiogenesis and lymphangiogenesis through inhibition of VEGF signaling in a murine sarcoma model. Oncol Rep 29:1907–1913
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25:501–515
Acknowledgements
We thank the members of Kansas City VA Research Office and Midwest Biomedical Research Foundation Administrative and clerical supports.
Funding
The work is supported by Merit review grant from Department of Veterans Affairs (Sushanta K. Banerjee, 5I01BX001989–04 and Snigdha Banerjee, I01BX001002–05), KUMC Lied Basic Science Grant Program (SKB), and Grace Hortense Greenley Trust, directed by The Research Foundation in memory of Eva Lee Caldwell (SKB).
Author information
Authors and Affiliations
Contributions
Conception and design: S. K. Banerjee, S. Banerjee I. Haque and G. Maity; Development of Methodology: G. Maity and A. Ghosh, I. Haque and S. Banerjee; Acquisition of Data: G. Maity and J. Chakraborty; Analysis and Interpretation of Data: G. Maity, A. Ghosh, J. Chakraborty, S. Banerjee and S.K. Banerjee; Writing and review of the manuscript: G. Maity, J. Chakraborty, S. Banerjee and S. K. Banerjee; Administrative, technical or material support: G. Maity, A. Ghosh and S. K. Banerjee, and Study supervision: S. K. Banerjee.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Compliance with ethical standard of VA Medical Center.
Consent for publication
All the authors of this manuscript have agreed to publish this article.
Competing interests
No potential conflicts of interest were disclosed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Maity, G., Chakraborty, J., Ghosh, A. et al. Aspirin suppresses tumor cell-induced angiogenesis and their incongruity. J. Cell Commun. Signal. 13, 491–502 (2019). https://doi.org/10.1007/s12079-018-00499-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-018-00499-y