Abstract
Background and aims
Persistent inflammatory response and immune activation are the core mechanisms underlying acute-on-chronic liver failure (ACLF). Previous studies have shown that deficiency of V-type immunoglobulin domain-containing suppressor of T-cell activation (VISTA) exacerbates the progression of inflammatory diseases. We aimed to clarify the role of VISTA in the pathogenesis of ACLF.
Methods
Blood and liver samples were collected from healthy subjects, stable cirrhosis, and ACLF patients to characterize VISTA expression and function. An ACLF mouse model was used to ascertain potential benefits of anti-VISTA monoclonal antibody (mAb) treatment.
Results
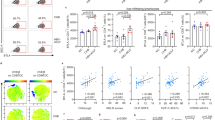
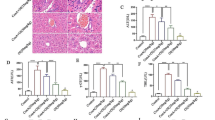
VISTA expression was significantly reduced in the naïve and central memory CD4+ T cells from patients with ACLF. The expression of VISTA on CD4+ T cells was associated with disease severity and prognosis. VISTA downregulation contributed to the activation and proliferation of CD4+ T cells and enhanced the differentiation of T helper 17 cells (Th17) and secretion of inflammatory cytokines through the activated Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway. Moreover, agonistic anti-VISTA mAb treatment inhibited the activation and cytokine production of CD4+ T cells and reduced mortality and liver inflammation of the ACLF mice.
Conclusions
The decreased expression of VISTA may facilitate development of Th17 cells and promote the progression of inflammation in ACLF patients. These findings are helpful for elucidating the pathogenesis of ACLF and for the identification of new drug targets.
Graphical abstract







Similar content being viewed by others
Availability of data and materials
All relevant data supporting the conclusions of this study are displayed within this manuscript. More detailed data is available from the corresponding author upon request.
References
Moreau R, Jalan R, Gines P, et al. CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437
Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3: 100176
Laleman W, Claria J, Van der Merwe S, et al. Systemic inflammation and acute-on-chronic liver failure: too much not enough. Can J Gastroenterol Hepatol. 2018;2018:1027152
Zhang GL, Xie DY, Lin BL, et al. Imbalance of interleukin-17-producing CD4 T cells/regulatory T cells axis occurs in remission stage of patients with hepatitis B virus-related acute-on-chronic liver failure. J Gastroenterol Hepatol. 2013;28:513–521
Li Q, Wang J, Lu M, et al. Acute-on-chronic liver failure from chronic-hepatitis-B, who is the behind scenes. Front Microbiol. 2020;11: 583423
Zhou X, Li Y, Ji Y, et al. PD-1 involvement in peripheral blood CD8+ T lymphocyte dysfunction in patients with acute-on-chronic liver failure. J Clin Transl Hepatol. 2021;9:283–290
Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592
Flies DB, Wang S, Xu H, et al. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187:1537–1541
Kunishige T, Taniguchi H, Ohno T, et al. VISTA is crucial for corneal allograft survival and maintenance of immune privilege. Invest Ophthalmol Vis Sci. 2019;60:4958–4965
Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J Clin Invest. 2014;124:1966–1975
Wang L, Le Mercier I, Putra J, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci U S A. 2014;111:14846–14851
Derakhshani A, Asadzadeh Z, Baradaran B, et al. The expression pattern of VISTA in the PBMCs of relapsing-remitting multiple sclerosis patients: a single-cell RNA sequencing-based study. Biomed Pharmacother. 2022;148: 112725
Hid Cadena R, Reitsema RD, Huitema MG, et al. Decreased expression of negative immune checkpoint VISTA by CD4+ T Cells Facilitates T Helper 1, T Helper 17, and T follicular helper lineage differentiation in GCA. Front Immunol. 2019;10:1638
Ceeraz S, Sergent PA, Plummer SF, et al. VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthritis Rheumatol. 2017;69:814–825
Sarin SK, Choudhury A, Sharma MK, et al. APASL ACLF Research Consortium (AARC) for APASL ACLF working party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390
Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;4:797–805
Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660
Jalan R, Saliba F, Pavesi M, et al. CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047
Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–149
Wang LY, Meng QH, Zou ZQ, et al. Increased frequency of circulating Th17 cells in acute-on-chronic hepatitis B liver failure. Dig Dis Sci. 2012;57:667–674
Xu C, Lu Y, Zheng X, et al. TLR2 expression in peripheral CD4+ T cells promotes Th17 response and is associated with disease aggravation of hepatitis B virus-related acute-on-chronic liver failure. Front Immunol. 2017;8:1609
Harris TJ, Grosso JF, Yen HR, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317
Xiang X, Feng D, Hwang S, et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020;72:736–745
Triantafyllou E, Woollard KJ, McPhail MJW, et al. The role of monocytes and macrophages in acute and acute-on-chronic liver failure. Front Immunol. 2018;9:2948
Bharaj P, Chahar HS, Alozie OK, et al. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS ONE. 2014;9: e109103
Rogers BM, Smith L, Dezso Z, et al. VISTA is an activating receptor in human monocytes. J Exp Med. 2021;218: e20201601
Bharaj P, Ye C, Petersen S, et al. Gene array analysis of PD-1H overexpressing monocytes reveals a pro-inflammatory profile. Heliyon. 2018;4: e00545
ElTanbouly MA, Zhao Y, Schaafsma E, et al. VISTA: a target to manage the innate cytokine storm. Front Immunol. 2021;11: 595950
ElTanbouly MA, Zhao Y, Nowak E, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science. 2020;367:eaay0524
Seto WK, Lai CL, Yuen MF. Acute-on-chronic liver failure in chronic hepatitis B. J Gastroenterol Hepatol. 2012;27:662–669
Xu W, Hiếu T, Malarkannan S, et al. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15:438–446
Li N, Xu W, Yuan Y, et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep. 2017;7:1485
Zhang M, Zhou L, Xu Y, et al. A STAT3 palmitoylation cycle promotes T17 differentiation and colitis. Nature. 2020;586:434–439
Nautiyal N, Maheshwari D, Tripathi DM, et al. Establishment of a murine model of acute-on-chronic liver failure with multi-organ dysfunction. Hepatol Int. 2021;15:1389–1401
Flies DB, Higuchi T, Chen L. Mechanistic assessment of PD-1H coinhibitory receptor-induced T cell tolerance to allogeneic antigens. J Immunol. 2015;194:5294–5304
Delaney B, Strom SC, Collins S, et al. Carbon tetrachloride suppresses T-cell-dependent immune responses by induction of transforming growth factor-beta 1. Toxicol Appl Pharmacol. 1994;126:98–107
Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342
Ramavath NN, Gadipudi LL, Provera A, et al. Inducible T-cell costimulator mediates lymphocyte/macrophage interactions during liver repair. Front Immunol. 2021;12: 786680
Guo TL, McCay JA, Brown RD, et al. Carbon tetrachloride is immunosuppressive and decreases host resistance to Listeria monocytogenes and Streptococcus pneumoniae in female B6C3F1 mice. Toxicology. 2000;154:85–101
Song LJ, Yin XR, Mu SS, et al. The differential and dynamic progression of hepatic inflammation and immune responses during liver fibrosis induced by schistosoma japonicum or carbon tetrachloride in mice. Front Immunol. 2020;11: 570524
Zhao S, Lu ZH, Cui JJ, et al. Nitroglycerin ameliorates liver injury and regulates adaptive immunity in mice. Drug Dev Res. 2020;81:557–563
Acknowledgement
We thank Dr. Haiyan Lv (Fudan University) for her helpful suggestions during the study.
Funding
This study was financially supported by the National Natural Science Foundation of China (81871640, 82172255), Shanghai Shen Kang Hospital Development Center (No. SHDC12019116), and Shanghai Key Clinical Specialty Construction Program (ZK2019B24).
Author information
Authors and Affiliations
Contributions
YZ and XYZ: made the study concept and design; statistical analysis and drafting of manuscript was done by YZ. XYZ, JJH and YFG: conducted some experimental studies; FFY, FHL and HXZ: helped experimental design; YZ, XYZ, and ZLS: conducted the collection of clinical data and sample; critical revision of manuscript done for important intellectual content was done by YXH, RCM and JMZ; JMZ: supervised the whole project. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The Yao Zhang, Xueyun Zhang, Jiajia Han, Yifei Guo, Feifei Yang, Fahong Li, Haoxiang Zhu, Zhongliang Shen, Yuxian Huang, Richeng Mao, Jiming Zhang have no conflicts of interest to declare.
Ethical approval
This study was conducted in compliance with the principles of the Helsinki Declaration and was approved by the Ethical Committee of Huashan Hospital of Fudan University and the Ethical Committee of the Shanghai Public Health Clinical Center.
Consent to participate
All of the patients provided a written informed consent.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhang, X., Han, J. et al. Downregulated VISTA enhances Th17 differentiation and aggravates inflammation in patients with acute-on-chronic liver failure. Hepatol Int 17, 1000–1015 (2023). https://doi.org/10.1007/s12072-023-10505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10505-0