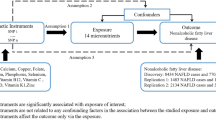
Abstract
Background
Whether supplementation with diet-derived antioxidants is beneficial for nonalcoholic fatty liver disease (NAFLD) is still controversial and we hope to answer this question using population-based genetic data.
Methods
A total of 8485 NAFLD cases and 658,849 healthy controls from four independent NAFLD genome-wide association studies were enrolled in this study. Genetic variants closely associated with the diet-derived antioxidants were selected to predict their circulating levels. A bi-directional Mendelian randomization (MR) design was employed to assess their causations.
Results
Genetic correlation analyses suggested inverse associations between diet-derived antioxidants and NAFLD. MR analyses indicated that the odds ratio (OR) of per standard deviation increase in genetically predicted toenail and blood selenium was 1.179 for NAFLD (95% confidence interval [1.083–1.284]). Also, the genetically elevated selenium level was causally associated with increased levels of C-reactive protein, fibrinogen, alkaline phosphatase and glycated hemoglobin. The OR of 1 µg/dL increase in genetically predicted serum lycopene was 1.082 (95%CI [1.051–1.113]). No other causal associations were found for NAFLD. However, we observed protective effects of genetically predicted β-carotene (OR = 0.929[0.911–0.947]) and retinol (OR = 0.483[0.460–0.508]) on type 2 diabetes (T2D), and further they could reduce the serum levels of blood lipids and glucose. Reverse MR analysis suggested genetically predicated NAFLD status would not affect the levels of diet-derived antioxidants.
Conclusion
Overall, we observed the positive associations of genetically predicted selenium and lycopene with NAFLD. However, the genetically predicted β-carotene and retinol levels were inversely associated with the risk of T2D.
Graphical Abstract






Similar content being viewed by others
Availability of data materials
All genome-wide association studies can be accessed via GWAS Catalog database (https://www.ebi.ac.uk/gwas/) and Open GWAS database (https://gwas.mrcieu.ac.uk/). The summary statistics of GWAS meta-analyses generated in this study can be accessible upon request.
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- MR:
-
Mendelian randomization
- SNP:
-
Single nucleotide polymorphism
- GWAS:
-
Genome-wide association study
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transferase
- ALP:
-
Alkaline phosphatase
- γ-GT:
-
γ-Glutamyl transferase
- T2D:
-
Type 2 diabetes
- LDSC:
-
Linkage disequilibrium score regression
- LD:
-
Linkage disequilibrium
- BMI:
-
Body mass index
- WHR:
-
Waist-to-hip ratio
- UKB:
-
UK biobank
- OR:
-
Odds ratio
- HbA1c:
-
Glycated hemoglobulin
- LDL:
-
Low-density lipoprotein
- TC:
-
Total cholesterol
- HDL:
-
High-density lipoprotein
- TG:
-
Triglycerides
- FI:
-
Fasting insulin
- STROBE-MR:
-
Strengthening the reporting of observational studies in epidemiology-Mendelian randomization
- eMERGE:
-
Electronic medical records and genomics
- GOLD:
-
Genetics of obesity-related liver disease
- IVW:
-
Inverse variance weighted
- FDR:
-
False discovery rate
- MRPRESSO:
-
Mendelian randomization pleiotropy residual sum and outlier
- IVs:
-
Instrumental variables
- NHANES:
-
National health and nutrition examination survey
References
Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999-2014.e1991
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922
Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558
He Z, Li X, Yang H, Wu P, Wang S, Cao D, et al. Effects of oral vitamin C supplementation on liver health and associated parameters in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Front Nutr. 2021;8: 745609
Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M. High serum carotenoids are associated with lower risk for developing elevated serum alanine aminotransferase among Japanese subjects: the Mikkabi cohort study. Br J Nutr. 2016;115:1462–1469
Ma C, Liu Y, He S, Zeng J, Li P, Ma C, et al. Negative association between antioxidant vitamin intake and non-alcoholic fatty liver disease in Chinese non-diabetic adults: mediation models involving superoxide dismutase. Free Radic Res. 2020;54:670–677
Xiao ML, Zhong HL, Lin HR, Liu CY, Yan Y, Ke YB, et al. Higher serum vitamin A is associated with a worsened progression of non-alcoholic fatty liver disease in adults: a prospective study. Food Funct. 2022;13:970–977
Jeon D, Son M, Shim J. Dynamics of serum retinol and alpha-tocopherol levels according to non-alcoholic fatty liver disease status. Nutrients. 2021;13:1720
Davies NM, Holmes MV, Davey SG. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362: k601
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580
Luo J, le Cessie S, van Heemst D, Noordam R. Diet-derived circulating antioxidants and risk of coronary heart disease: a Mendelian randomization study. J Am Coll Cardiol. 2021;77:45–54
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241
Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295
Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, et al. A global reference for human genetic variation. Nature. 2015;526:68–74
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375: n2233
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326:1614–1621
Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7: e1001324
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–389
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698
Wang X, Seo YA, Park SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: national health and nutrition examination survey (NHANES) 2011–2016. Environ Res. 2021;197:111190
Yang Z, Yan C, Liu G, Niu Y, Zhang W, Lu S, et al. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: a cross-sectional analysis. Sci Rep. 2016;6:37288
Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, et al. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study. J Am Coll Nutr. 2020;39:103–111
Urbano T, Filippini T, Lasagni D, De Luca T, Grill P, Sucato S, et al. Association of urinary and dietary selenium and of serum selenium species with serum alanine aminotransferase in a healthy Italian population. Antioxidants (Basel). 2021;10:1516
Park K, Rimm EB, Siscovick DS, Spiegelman D, Manson JE, Morris JS, et al. Toenail selenium and incidence of type 2 diabetes in U.S. male and female. Diabetes Care. 2012;35:1544–1551
Lazard M, Dauplais M, Blanquet S, Plateau P. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. Biomol Concepts. 2017;8:93–104
Li J, Yu J, Yang J, Cui J, Sun Y. Dietary iron and zinc intakes and nonalcoholic fatty liver disease: a meta-analysis. Asia Pac J Clin Nutr. 2021;30:704–714
Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18
Song X, Luo Y, Ma L, Hu X, Simal-Gandara J, Wang LS, et al. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin Cancer Biol. 2021;73:331–346
Xiao ML, Chen GD, Zeng FF, Qiu R, Shi WQ, Lin JS, et al. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: a prospective study. Eur J Nutr. 2019;58:721–730
Iqbal WA, Mendes I, Finney K, Oxley A, Lietz G. Reduced plasma carotenoids in individuals suffering from metabolic diseases with disturbances in lipid metabolism: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr. 2021;72:879–891
Chen X, Zhao Y, Liu K, Li Z, Tan X, Wang Y, et al. Lycopene aggravates acute gastric injury induced by ethanol. Front Nutr. 2021;8: 697879
Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF. Association between dietary vitamin c intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS One. 2016;11: e0147985
Zheng JS, Luan J, Sofianopoulou E, Imamura F, Stewart ID, Day FR, et al. Plasma vitamin C and type 2 diabetes: genome-wide association study and Mendelian randomization analysis in European populations. Diabetes Care. 2021;44:98–106
Lawlor DA, Davey Smith G, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet. 2004;363:1724–1727
Lee SW, Baek SM, Kang KK, Lee AR, Kim TU, Choi SK, et al. Vitamin C deficiency inhibits nonalcoholic fatty liver disease progression through impaired de novo lipogenesis. Am J Pathol. 2021;191:1550–1563
Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481–1488
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668
Wallström P, Wirfält E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr. 2001;73:777–785
Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73:263–276
Sun YQ, Burgess S, Staley JR, Wood AM, Bell S, Kaptoge SK, et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear Mendelian randomisation analyses. BMJ. 2019;364: l1042
Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet. 2020;52:740–747
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study. We want to thank MRC-IEU and Neale Lab for making UK Biobank GWAS summary statistics openly available. Besides, we would like to thank all the other investigators for making summary statistics openly available.
Funding
The work is supported by the Financial Department of Jilin Province (Grant NO. JLSWSRCZX2021-026; Grant NO. JLSWSRCZX2020-045; Grant NO. 2018SCZWSZX-033).
Author information
Authors and Affiliations
Contributions
GL and LC proposed and designed the research. LC undertook data collection and performed the main data analysis. ZF checked the statistical analysis. LC and ZF wrote the draft of the manuscript. XS and WQ were responsible for reviewing and substantially revising the manuscript. WM visualized the results and revised the manuscript. KC and YC were responsible for reviewing the statistical methods. GW provided necessary advice and revised the manuscript. GL supervised the whole research and claimed the integrity of data analysis.
Corresponding author
Ethics declarations
Conflict of interest
Lanlan Chen, Zhongqi Fan, Xiaodong Sun, Wei Qiu, Wentao Mu, Kaiyuan Chai, Yannan Cao, Guangyi Wang and Guoyue Lv declare that they have no conflicts of interest.
Ethics approval and consent to participate
Each genome-wide association study (GWAS) has been approved by its corresponding Ethical Review Committee. This study was exempted from the Ethical Review Committee of the First Hospital of Jilin University since all GWAS summary statistics are openly available and can be used and analyzed without restriction except for identifying the individual-level information.
Consent for publication
Each author has given his/her consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Fan, Z., Sun, X. et al. Diet-derived antioxidants and nonalcoholic fatty liver disease: a Mendelian randomization study. Hepatol Int 17, 326–338 (2023). https://doi.org/10.1007/s12072-022-10443-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10443-3