Abstract
Background
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a newly proposed definition of fatty liver disease (FLD) independent of excessive alcohol consumption (EAC) and hepatitis viral infection. Evidence on the mortality risk in different types of FLD [nonalcoholic FLD (NAFLD), alcoholic FLD (AFLD), and MAFLD] is sparse, hindering the identification of high-risk populations for preferential clinical surveillance.
Methods
A total of 11,000 participants in the Third National Health and Nutrition Examination Survey were enrolled. Participants were categorized into three groups [FLD( − ), MAFLD( − ), and MAFLD( +)] according to FLD and MAFLD criteria, and further categorized into six groups by EAC. Multivariate Cox proportional hazard model was used to estimate the risk of all-cause, cardiovascular-related, and cancer-related mortality.
Results
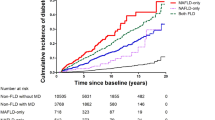
During a median follow-up of 23.2 years, a total of 3240 deaths were identified. Compared with FLD( − )/EAC( − ) participants, MAFLD( +) individuals had higher all-cause mortality risk [hazard ratio (HR) = 1.28, 95% confidence interval (CI) = 1.18–1.39] regardless of EAC status [MAFLD( +)/NAFLD: HR = 1.22, 95%CI = 1.11–1.34; MAFLD( +)/AFLD: HR = 1.83, 95%CI = 1.46–2.28], while not for MAFLD( − ) individuals. Furthermore, diabetes-driven-MAFLD had higher mortality risk (HR = 2.00, 95%CI = 1.77–2.27) followed by metabolic dysregulation-driven-MAFLD (HR = 1.30, 95%CI = 1.06–1.60) and overweight/obesity-driven-MAFLD (HR = 1.11, 95%CI = 1.00–1.22). Additionally, MAFLD( − ) participants with elevated fibrosis score were also associated with statistically significantly higher mortality risk (HR = 3.23, 95%CI = 1.63–6.40).
Conclusions
Utilizing a representative sample of the US population, we proved the validity of MAFLD subtype and fibrosis score, rather than the traditional definition (NAFLD and AFLD), in the risk stratification of FLD patients. These findings may be applied to guide the determination of surveillance options for FLD patients.





Similar content being viewed by others
Data availability
The Third National Health and Nutrition Examination Survey (NHANES III) dataset are publicly available at National Center for Health Statistics of the Center for Disease Control and Prevention and the link to the database is https://wwwn.cdc.gov/nchs/nhanes/Nhanes3/Default.aspx.
Abbreviations
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- FLD:
-
Fatty liver disease
- NAFLD:
-
Nonalcoholic fatty liver disease
- AFLD:
-
Alcoholic fatty liver disease
- T2DM:
-
Type 2 diabetes mellitus
- NHANES:
-
National health and nutrition examination survey
- NCHS:
-
National center for health statistics
- HSUE:
-
Hepatic steatosis ultrasound examination
- EAC:
-
Excessive alcohol consumption
- APRI:
-
Aspartate aminotransferase to platelet ratio index
- FIB-4:
-
Fibrosis-4
- NFS:
-
NAFLD fibrosis score
- NDI:
-
National death index
- ICD-9:
-
International classification disease-ninth
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- HDL-C:
-
High-density lipoprotein cholesterol
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
References
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209
Prasoppokakorn T, Pitisuttithum P, Treeprasertsuk S. Pharmacological therapeutics: current trends for metabolic dysfunction-associated fatty liver disease (MAFLD). J Clin Transl Hepatol. 2021;9(6):939–946
Le P, Chaitoff A, Rothberg MB, McCullough A, Gupta NM, Alkhouri N. Population-based trends in prevalence of nonalcoholic fatty liver disease in US adults with type 2 diabetes. Clin Gastroenterol Hepatol. 2019;17(11):2377–2378
Lin H, Zhang X, Li G, Wong GL, Wong VW. Epidemiology and clinical outcomes of metabolic (dysfunction)-associated fatty liver disease. J Clin Transl Hepatol. 2021;9(6):972–982
Chang Y, Cho YK, Cho J, Jung HS, Yun KE, Ahn J, et al. Alcoholic and nonalcoholic fatty liver disease and liver-related mortality: a cohort study. Am J Gastroenterol. 2019;114(4):620–629
Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–347
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-Results from NHANES III. Front Med. 2021;8: 693507
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–1291
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172–81.e6
Chen X, Chen S, Pang J, Tang Y, Ling W. Are the different MAFLD subtypes based on the inclusion criteria correlated with all-cause mortality? J Hepatol. 2021;75(4):987–989
Semmler G, Wernly S, Bachmayer S, Leitner I, Wernly B, Egger M, et al. Metabolic dysfunction-associated fatty liver disease (MAFLD)-rather a bystander than a driver of mortality. J Clin Endocrinol Metab. 2021;106(9):2670–2677
Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, et al. Metabolic dysfunction-associated fatty liver disease and mortality among Chinese adults: a prospective cohort study. J Clin Endocrinol Metab. 2021;107(2):e745–e755
Moon JH, Kim W, Koo BK, Cho NH. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut Liver. 2022;16(3):433-442. https://doi.org/10.5009/gnl210167
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315
Wang Z, Bertot LC, Jeffrey GP, Joseph J, Garas G, de Boer B, et al. Serum fibrosis tests guide prognosis in metabolic dysfunction-associated fatty liver disease patients referred from primary care. Clin Gastroenterol Hepatol. 2021;S1542–3565(21):01055–01057
Tang LJ, Ma HL, Eslam M, Wong GL, Zhu PW, Chen SD, et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabolism. 2021;128: 154958
Decraecker M, Dutartre D, Hiriart JB, Irles-Depé M, Chermak F, Foucher J, et al. Long-term prognosis of patients with metabolic (dysfunction)-associated fatty liver disease by non-invasive methods. Aliment Pharmacol Ther. 2022;55(5):580–592
CDC/NCHS. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey NHANES III (1988–94). Hyattsville: National Center for Health Statistics Centers for Disease Control and Prevention; 1996
Third National Health and Nutrition Examination Survey. Hepatic/Gallbladder Ultrasound and Hepatic Steatosis (HGUHS). Available at: https://wwwn.cdc.gov/nchs/Data/Nhanes3/34A/HGUHS.htm. Accessed October 14, 2020.
National Health; and Nutrition Examination Survey (NHANES) III. Hepatic Steatosis Ultrasound Images Assessment Procedures Manual 2010. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf. Accessed October 15, 2020.
Pearson MM, Kim NJ, Berry K, Moon AM, Su F, Vutien P, et al. Associations between alcohol use and liver-related outcomes in a large national cohort of patients with cirrhosis. Hepatol Commun. 2021;5(12):2080–2095
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2021;S1542–3565(21):01276–01283
Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37(7):936–949
Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74(12):737–748
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344
Shahid RK, Ahmed S, Le D, Yadav S. Diabetes and cancer: risk, challenges, management and outcomes. Cancers. 2021;13(22):5735
Dongiovanni P, Valenti L. A nutrigenomic approach to non-alcoholic fatty liver disease. Int J Mol Sci. 2017;18(7):1534
Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, Global BMI Mortality Collaboration, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786
Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11–12):1260–1270
Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19(10):2138–47.e10
Wong VW, Lazarus JV. Prognosis of MAFLD vs NAFLD and implications for a nomenclature change. J Hepatol. 2021;75(6):1267–1270
Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090
Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–1019
Acknowledgements
We would like to thank the participants and staff of the National Health and Nutrition Examination Survey (NHANES) for their valuable contributions. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2021YFC2500400), the Beijing-Tianjin-Hebei Basic Research Cooperation Special Project (20JCZXJC00090), the Tianjin Municipal Commission of Health and Wellness Project (TJWJ2021MS008), and the Doctoral Fund of Tianjin Medical University Cancer Institute and Hospital (B2013). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
KXC has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Study concept and design: YCZ, ZYL, and KXC. Acquisition of data: YCZ, ZYL, BM, LML, WW, and CS. Analysis and interpretation of data: YCZ, ZYL, CS, HJD, YBH, BM, LML, and WW. Drafting of the manuscript: YCZ, ZYL, and KXC. Critical revision of the manuscript for important intellectual content: YCZ, ZYL, WW, HJD, YBH, FFS, FJS, and KXC. Obtained funding: ZYL, YBH, and KXC. Administrative, technical, or material support: HJD, YBH, FFS, FJS, and KXC. Study supervision: HJD, YBH, FFS, FJS, and KXC.
Corresponding author
Ethics declarations
Conflict of interest
The authors (Ya‑Cong Zhang, Zhang‑Yan Lyu, Bing Ma, Li‑Min Li, Wei Wang, Chao Sheng, Hong‑Ji Dai, Yu‑Bei Huang, Fang‑Fang Song, Feng‑Ju Song, Ke‑Xin Chen) declare that they have no conflict of interest.
Animal research
Not applicable.
Consent to participate
The Third National Health and Nutrition Examination Survey (NHANES III) was approved by the institutional reviews board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/irba98.htm), and written informed consent was obtained from all individual participants in the study.
Consent to publish
All authors of this manuscript have read and approved the final submitted version and are aware that they are listed as an author on this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, YC., Lyu, ZY., Ma, B. et al. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hepatol Int 16, 835–845 (2022). https://doi.org/10.1007/s12072-022-10362-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10362-3