Abstract
Background/purpose
Conflicting results have been reported between the use of extracellular contrast agent (ECA) and hepatobiliary contrast agent (HBA) when magnetic resonance imaging (MRI) is used for the diagnosis of hepatocellular carcinoma (HCC). Therefore, we aimed to compare the diagnostic performance of MRI using ECA (ECA-MRI) and HBA (HBA-MRI).
Methods
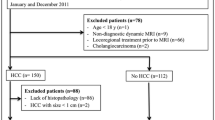
Original studies reporting the diagnostic performance of contrast-enhanced MRI for the diagnosis of HCC published between January 2010 and February 2020 were identified in a Pubmed, EMBASE, and Cochrane Library database search. The pooled sensitivity and specificity of ECA-MRI and HBA-MRI were calculated using a bivariate random effects model and compared using a joint-model bivariate meta-regression. Subgroup analyses were performed to compare the diagnostic performance of ECA-MRI and HBA-MRI according to study design, underlying liver disease, lesion size, reference standard, and imaging criteria.
Results
Of the 1760 screened articles, 31 studies were included: 15 studies included 2890 lesions imaged using ECA-MRI and 19 studies included 3893 lesions imaged using HBA-MRI. The pooled sensitivity and specificity were not significantly different between ECA-MRI (sensitivity, 72% [95% confidence interval 65–79%]; specificity 92% [89–95%]) and HBA-MRI (76% [68–83%]; 92% [87–95%], p = 0.72). Subgroup analyses did not find differences in diagnostic performance between ECA-MRI and HBA-MRI according to study design (p ≥ 0.11), underlying disease (p ≥ 0.09), lesion size (≤ 2 cm, p = 0.97), reference standard (p = 0.70), or imaging criteria (p = 0.33).
Conclusion
ECA-MRI showed similar performance to HBA-MRI in the diagnosis of HCC. The contrast agent might be selected with consideration of the advantages of each agent.



Similar content being viewed by others
References
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750
Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014;273:30–50
Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015;275:97–109
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370
Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol 2015;25:2859–2868
Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 2018;67:401–421
Min JH, Kim JM, Kim YK, Kang TW, Lee SJ, Choi GS, et al. Prospective intraindividual comparison of magnetic resonance imaging with gadoxetic acid and extracellular contrast for diagnosis of hepatocellular carcinomas using the liver imaging reporting and data system. Hepatology 2018;68:2254–2266
Paisant A, Vilgrain V, Riou J, Oberti F, Sutter O, Laurent V, et al. Comparison of extracellular and hepatobiliary MR contrast agents for the diagnosis of small HCCs. J Hepatol 2020;72:937–945.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-W94
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536
Leeflang MM, Deeks JJ, Rutjes AW, Reitsma JB, Bossuyt PM. Bivariate meta-analysis of predictive values of diagnostic tests can be an alternative to bivariate meta-analysis of sensitivity and specificity. J Clin Epidemiol 2012;65:1088–1097
Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9
van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–893
Min JH, Kim JM, Kim YK, Cha DI, Kang TW, Kim H, et al. Magnetic resonance imaging with extracellular contrast detects hepatocellular carcinoma with greater accuracy than with gadoxetic acid or computed tomography. Clin Gastroenterol Hepatol 2020;18:2091–2100
Lee S, Kim MJ, Kim SS, Shin H, Kim DY, Choi JY, et al. Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol Int 2020;14:70–79
Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, Choi BI. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 2014;271:748–760
Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 2013;266:452–461
Joo I, Lee JM, Lee DH, Jeon JH, Han JK. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol 2019;29:1724–1732
Chernyak V, Fowler KJ, Heiken JP, Sirlin CB. Use of gadoxetate disodium in patients with chronic liver disease and its implications for liver imaging reporting and data system (LI-RADS). J Magn Reson Imaging 2019;49:1236–1252
Zhang W, Wang X, Miao Y, Hu C, Zhao W. Liver function correlates with liver-to-portal vein contrast ratio during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MR at 3 Tesla. Abdom Radiol (NY) 2018;43:2262–2269
Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371–1382
Kang TW, Kong SY, Kang D, Kang MW, Kim YK, Kim SH, et al. Use of gadoxetic acid-enhanced liver MRI and mortality in more than 30,000 patients with hepatocellular carcinoma: a nationwide analysis. Radiology 2020;295:114–124
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. New York: Wiley; 2009
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Grant No. NRF-2019R1G1A1099743) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI18C2383).
Author information
Authors and Affiliations
Contributions
SHC and SYK contributed to the study concept and design. DWK and SHC acquired and analyzed the data. SHC, SHP, and KWK performed the statistical analysis. DWK and SHC drafted the manuscript. SYK, JHB, and SSL made critical revisions to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sang Hyun Choi receives research funding from Bayer Healthcare. The other authors (Dong Wook Kim, So Yeon Kim, Jae Ho Byun, Seung Soo Lee, Seong Ho Park and Kyung Won Kim) have no conflicts of interest to declare.
Statement of ethics
Institutional Review Board approval was not required due to the study design, which was a systemic review and meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, D.W., Choi, S.H., Kim, S.Y. et al. Diagnostic performance of MRI for HCC according to contrast agent type: a systematic review and meta-analysis. Hepatol Int 14, 1009–1022 (2020). https://doi.org/10.1007/s12072-020-10100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10100-7