Abstract
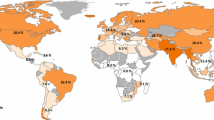
Hepatitis C genotype 3 (GT-3) infection comprises up to 30 % of all HCV infections worldwide, with roughly 54.3 million cases concentrated in South and Southeast Asia. Longitudinal studies have demonstrated significantly increased rates of steatosis, fibrosis, and hepatocellular carcinoma in GT-3 disease, thus distinguishing this genotype as both the most difficult and urgent to treat. However, novel direct-acting antiviral agents currently approved have not demonstrated the uniform potency seen in GT-1 disease for GT-3. This review outlines (1) the epidemiology and natural history, (2) phase II and III clinical trials demonstrating effective treatment options, and (3) current and future therapeutic issues in the quest to eradicate hepatitis C genotype 3 disease.

Similar content being viewed by others
References
Gower E et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(1 Suppl):S45–S57
Bruggmann P et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat 2014;21:5–33
Messina JP et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015;61.1: 77–87
Agnello V et al. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA 1999;96 12766–12771
Shi ST et al. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology 2002;292(2):198–210
Serfaty L et al. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol 2001;34:428–434
Jackel-Cram C et al. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol 2007 46(6):999–1008
Jackel-Cram C et al. Hepatitis C virus genotype-3a core protein enhances sterol regulatory element-binding protein-1 activity through the phosphoinositide 3-kinase-Akt-2 pathway. J Gen Virol 2010;91(Pt 6):1388–1395
Rubbia-Brandt L et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype. J Hepatol 2000;33:106–115
Bochud PY et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009;51(4):655–666
Probst A et al. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J Viral Hepat 2011;18(11):745–759
Kanwal F et al. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of US Veterans with HCV. Hepatology 2014;60(1):98–105
Nkontchou G et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat 2011;18(10):e516–e522
Sood A et al. Comparison of low-dose pegylated interferon versus standard high-dose pegylated interferon in combination with ribavirin in patients with chronic hepatitis C with genotype 3: an Indian experience. J Gastroenterol Hepatol 2008;23(2): 203–207
Ferenci P et al. A randomized, prospective trial of ribavirin 400 mg/day versus 800 mg/day in combination with peginterferon alfa-2a in hepatitis C virus genotypes 2 and 3. Hepatology 2008;47(6): 1816–1823
Diago M et al. Identifying hepatitis C virus genotype 2/3 patients who can receive a 16-week abbreviated course of peginterferon alfa-2a (40KD) plus ribavirin. Hepatology 2010;51(6): 1897–1903
Mangia A et al. Individualized treatment with combination of Peg-interferon alpha 2b and ribavirin in patients infected with HCV genotype 3. J Hepatol 2010;53(6): 1000–1005
Manns M et al. Reduced dose and duration of peginterferon alfa-2b and weight-based ribavirin in patients with genotype 2 and 3 chronic hepatitis C. J Hepatol 2011;55(3): 554–563
Fernandez-Rodriguez CM et al. Randomized clinical trial comparing high versus standard dose of ribavirin plus peginterferon alfa-2a in hepatitis C genotype 3 and high viral load. Dargen-3 study. Gastroenterol Hepatol 2014;37(1): 1–8
Shoeb D et al. Extended duration therapy with pegylated interferon and ribavirin for patients with genotype 3 hepatitis C and advanced fibrosis: final results from the STEPS trial. J Hepatol 2014;60(4): 699–705
Foster GR et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 2011;141(3): 881–889 e881
Gane EJ et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368(1):34–44
Lawitz E et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 2013;13:401–408
Dore GJ et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology 2015;148(2): 355–366 e351
Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015;149(6):1462–1470
Lawitz E et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368(20): 1878–1887
Jacobson IM et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368(20): 1867–1877
Zeuzem S et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370(21): 1993–2001
Sulkowski MS et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014;312(4):353–361
Sulkowski MS et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370(3): 211–221
Nelson DR et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015;61(4):1127–1135
Gane EJ, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015;149(6):1454–1461
Gilead Sciences Inc. SOVALDI product monograph. Foster City; 2013
Gilead Sciences Inc. Harvoni (ledipasvir/sofosbuvir) Product monograph. Foster City; 2014
Product Information: Daklinza oral film-coated tablets, daclatasvir oral film-coated tablets. Bristol-Myers Squibb Pharma EEIG (per EMA), Uxbridge, UK, 2014
Kumar D et al. Hepatitis C virus genotype 3 is cytopathic to hepatocytes. Genotype-specific reversal of hepatic steatosis after sustained response to antiviral therapy. Hepatology 2002;36:1266–1272
Poynard T et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 2003;38:75–85
Shah SR et al. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin Gastroenterol Hepatol 2011;9(8):688–693
Gane EJ et al. Once-daily sofosbuvir with GS-5816 for 8 weeks with or without ribavirin in patients with HCV genotype 3 without cirrhosis result in high rates of SVR12: The ELECTRON-2 Study. Program and abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Mass. Abstract 79
Tran TT, Morgan TR, Thuluvath PJ, et al. Safety and efficacy of treatment with sofosbuvir + GS-5816 ± ribavirin for 8 or 12 weeks in treatment-naive patients with genotype 1-6 HCV infection. Program and abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Mass. Abstract 80
Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, et al. Sofosbuvir plus Velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 Hepatitis C virus infection: a randomized trial. Ann Intern Med 2015;163(11):809–817
Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al. Sofosbuvir and Velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373(27):2608–2617
Zhao Y et al. ACH-3422, A novel HCV NS5B RNA polymerase nucle- otide inhibitor, demonstrates improved potency over sofosbuvir against HCV genotype-3 replicons in vitro. Program and abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Mass. Abstract 1978
Asante-Appiah E et al. MK-8408, A potent and selective NS5A inhibitor with a high genetic barrier to resistance and activity against HCV genotypes 1-6. Program and abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Mass. Abstract 1979
Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E, Brainard D, Subramanian GM, McHutchison JG, Puoti M, Rockstroh JK et al. PHOTON-2 study team. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 21;385(9973):1098–1106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sarah Kattakuzhy, Rachel Levy, Elana Rosenthal, Lydia Tang, Eleanor Wilson and Shyam Kottilil declare that they do not have any financial or other conflicts of interest. This article is a review of clinical trial data and did not conduct any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Kattakuzhy, S., Levy, R., Rosenthal, E. et al. Hepatitis C genotype 3 disease. Hepatol Int 10, 861–870 (2016). https://doi.org/10.1007/s12072-016-9748-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9748-z