Abstract
Aim
The protective role of invariant natural killer T cells (iNKTs) against hepatitis B virus (HBV) infection remains controversial. We sought to clarify the role of peripheral iNKT cells during chronic HBV infection.
Methods
Sixty patients with chronic HBV infection were categorized into an immune tolerance phase (HBV-IT) (n = 16), an immune clearance phase (HBV-IC) (n = 19) and an inactive carrier phase (HBV-IA) (n = 25). Twenty healthy individuals were enrolled as healthy controls. Another 21 HBeAg-positive patients were administrated with entecavir (0.5 mg/day) for 6 months. The percentages of circulating iNKT cells and their IFN-γ and IL-4 expression levels were examined by flow cytometry. The relationships between serum HBV DNA, ALT levels, the percentages of iNKT cells, and their IFN-γ and IL-4 levels were analyzed.
Results
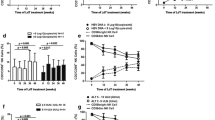
Compared to healthy controls, the percentage of iNKT cells decreased in HBV-IT, but increased in HBV-IC and HBV-IA. Circulating IFN-γ-producing iNKT cells gradually increased, whereas IL-4-producing iNKT cells gradually decreased from HBV-IT stage to HBV-IC and HBV-IA stages. The frequency of iNKT cells and their IFN-γ levels were reversely correlated with viral load. The levels of IL-4 expressed by iNKT cells were positively correlated to viral load and the serum ALT levels. After anti-virus therapy, the percentage of IFN-γ-producing iNKT cells increased while the percentage of IL-4-producing iNKT cells decreased.
Conclusions
During chronic HBV infection, the percentages of peripheral iNKT cells and its cytokines expressions of IFN-γ and IL-4 showed dynamic changes. The expression levels of IFN-γ and IL-4 were correlated with the clearance of HBV and liver injury.




Similar content being viewed by others
References
Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–10.
Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20.
Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007;45:507–39.
Pan CQ, Zhang JX. Natural history and clinical consequences of hepatitis B virus infection. Int J Med Sci 2005;2:36–40.
Shi YH, Shi CH. Molecular characteristics and stages of chronic hepatitis B virus infection. World J Gastroenterol 2009;15:3099–105.
Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76.
Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–25.
Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5:215–29.
Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 1997;15:535–62.
Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med 1994;180:1171–6.
Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med 1994;180:1097–106.
Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today 2000;21:573–83.
Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol 2002;2:557–68.
Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 2007;5:405–17.
Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med 2012;18:1060–8.
Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci USA 2007;104:18187–92.
Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005;23:877–900.
Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med 2005;202:1623–6.
Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007;25:297–336.
Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology 2014;58:1474–85.
Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009;86:513–28.
Yin S, Wang H, Bertola A, Feng D, Xu MJ, Wang Y et al. Activation of invariant natural killer T cells impedes liver regeneration by way of both IFN-gamma- and IL-4-dependent mechanisms. Hepatology 2014;60:1356–66.
Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 2009;49:1683–94.
Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 2000;192:921–30.
Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, Scheper RJ et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther 2009;14:809–18.
de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol 2004;173:1417–25.
Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S et al. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw 2005;16:283–8.
Kinjo Y, Kronenberg M. V alpha14 i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol 2005;25:522–33.
Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993;362:758–61.
Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 1998;187:1383–93.
Jiang X, Zhang M, Lai Q, Huang X, Li Y, Sun J et al. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One 2011;6:e28871.
Wang H, Park O, Gao B NKT cells in liver fibrosis: controversies or complexities. J Hepatol 2011;55:1166; author reply 1166–1167.
Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol 2011;54:1195–204.
Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 2011;53:219–29.
Minami K, Yanagawa Y, Iwabuchi K, Shinohara N, Harabayashi T, Nonomura K et al.. Negative feedback regulation of T helper type 1 (Th1)/Th2 cytokine balance via dendritic cell and natural killer T cell interactions. Blood 2005;106:1685–93.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81202662 and 81473477], and Science Research Project of Twelve Five-year Plan [2012ZX10005004-002], and Doctoral Fund of Ministry of Education of China [20123107110003], and Construction project of TCM clinical research base of State Administration of TCM China [JDZX2012058], and Shanghai Rising-Star Program [13QA1403500], and Three-year action plan of development of TCM in Shanghai [ZYSNXD-CC-ZDYJ015 and ZY3-CCCX-3-3027], and Training plan of outstanding young medical talents, Shanghai Municipal Health Bureau [XYQ2013093].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors concur with the submission and the authors have no potential conflicting interests.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). This study was approved by the local ethics committee, and all patients provided their written informed consent according to a protocol reviewed and approved by the Institutional Review Board of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine. All of these patients and normal controls were Chinese. Our study was approved by the local ethics committee, and all patients provided their written informed consent according to a protocol reviewed and approved by the Institutional Review Board of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Zhen-Hua Zhou has contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12072_2015_9650_MOESM2_ESM.tif
Figure 1 Dynamic changes of peripheral iNKTs and its cytokines expressions during chronic HBV infection. Circulating IFN-γ-producing iNKT cells gradually increased, whereas IL-4-producing iNKT cells gradually decreased from HBV- IT stage to HBV- IC and HBV -IA stages. The frequency of iNKT cells and their IFN-γ levels were reversely correlated to viral load. The levels of IL-4 expressed by iNKT cells were positively correlated to viral load and the serum ALT levels. Supplementary material 2 (TIFF 28 kb)
Rights and permissions
About this article
Cite this article
Li, M., Zhou, ZH., Sun, XH. et al. The dynamic changes of circulating invariant natural killer T cells during chronic hepatitis B virus infection. Hepatol Int 10, 594–601 (2016). https://doi.org/10.1007/s12072-015-9650-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-015-9650-0