Abstract
Background: Covid-19 infection increases the risk of opportunistic infections like mucormycosis. Cutaneous mucormycosis can occur primarily by direct inoculation or secondary to involvement of the underlying structures. Cutaneous manifestations include tender, erythematous, indurated lesions and necrotic plaques. As the disease evolves, cutaneous features manifest progressively. Objectives: To study the manifestations of the cutaneous signs of sinonasal mucormycosis and management of such cases based on severity of involvement. Materials and methods: A retrospective analysis of 21 patients with diagnosis of cutaneous mucormycosis secondary to sinonasal involvement was done with assessment of their skin lesion, area involved and their clinical stage being noted at the time of admission and after 24 h. Treatment consisted of combination of surgical debridement, daily dressing and liposomal amphotericin B. Observations and Results: Out of total 21 patients, there were 10 males and 11 females. Among risk factors, 14 cases had history of covid 19 infection, 5 had history of steroid intake, 6 had history of ICU stay and all had deranged blood sugar levels. Among disease prognosis, excellent outcomes appeared in stage I and stage III showed worst outcome. Conclusion: Since initial clinical presentation is similar to cellulitis and other soft-tissue infections, early recognition is difficult. In this cohort, the prognosis of secondary cutaneous mucormycosis remained poor, especially in ICU patients and those with numerous predisposing factors. Such patients presented in late stages of the disease and mortality rate was very high in such group.
Similar content being viewed by others
Introduction
India has visualized a recent surge in mucormycosis cases. Mucormycosis is an angioinvasive fungal infection. Multiple clinical presentations have been described in literature including rhino-orbital, cerebral, pulmonary, gastro-intestinal, and few rare forms like cutaneous, endocarditis and osteomyelitis [1].
“Mycosis mucorina” was coined by Arnold Paltauf in 1885 during his description of a case of disseminated mucormycosis [2]. This later came to be known as mucormycosis. It is a systemic fungal infection more prevalent in developing countries. The fungi of the order mucorales belonging to the class zygomycetes namely rhizopus, absidia and mucor have been identified with this disease [3]. The global incidence of this disease is on the rise post covid-19 pandemic. It is more frequent in an immunocompromised host, with uncontrolled diabetes being the most important risk factor. There is increasing evidence that coronavirus disease 2019 (COVID-19) may trigger new-onset diabetes mellitus (DM) [4]. Inappropriate use of steroids, low blood oxygen levels, prolonged ICU stay with poor air filtration, non-sterile medical devices, irrational use of broad spectrum antibiotics and antifungals (voriconazole) were also implicated in COVID-19 associated mucormycosis.
Cutaneous mucormycosis may be primary (acquired by direct inoculation during trauma, contaminated dressings, surgery, burns) or secondary. Secondary cutaneous mucormycosis occurs along with an underlying rhinocerebral, sinonasal or disseminated infection and is reported more frequently than the primary form. Keeping in view the growing incidence of cutaneous mucormycosis, the present study was undertaken. A retrospective analysis of patients with secondary cutaneous mucormycosis after rhino-cerebro-orbital involvement that were treated in our institute in the past one year was carried out. We divided our patients according to the severity of involvement. This categorisation helped us to determine the severity and prognosis in patients with cutaneous mucormycosis secondary to rhinocerebral involvement. It also helped to assess the rapid progression and optimal treatment according to stage for the same. To the best of our knowledge, after researching the existing literature, this is the largest case series of secondary cutaneous mucormycosis.
Objectives
To study the manifestations of the cutaneous signs of sinonasal mucormycosis and management of such cases based on severity of involvement.
Materials and Methods
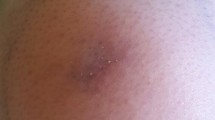
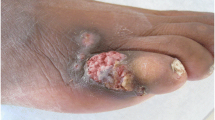
This was a retrospective study of 21 patients with the diagnosis of cutaneous mucormycosis secondary to rhinocerebral involvement. The cases which were confirmed histopathologically through observing non-septate or minimally septated broad, ribbon-like hyphae (10 to 20 micrometers) invading blood vessels, causing thrombosis, infarction and necrosis were included in the study. The available records of the patients were retrieved and analysed. The patients’ age, sex, detailed history with special emphasis on history of diabetes mellitus, Covid-19 infection, use of steroids and oxygen support were noted. Physical examination included the assessment of type of skin lesion, measurement of the area involved, endoscopic assessment of the nose and paranasal sinuses, inspection of the palate and orbit. Documented clinical signs from the time of initial assessment and subsequently every 24 h until the time of operative debridement were taken into consideration. Photographic records were also reviewed when available.(Figures 1a and b and 2).
Computed tomographic scan of nose and paranasal sinuses and contrast enhanced magnetic resonance imaging studies of these patients were reviewed.(Fig. 3) The protocol of the investigations and surgery was in accordance with the hospital norms. The patients were investigated and operated without delay after obtaining written informed consent from each participant or each participant’s guardian. A staging system (Table 1) was envisaged to facilitate separation of the patients into more refined subgroups and to provide a novel means for evaluating the prognosis.
Observations and Resultsl
Out of total number of patients (n = 21), there were 10 males and 11 females. The mean age of our patients was 53.3 years (range 20–76 years). Patients were categorised into various stages based on their clinical findings with which they presented to us with early lesions being categorized into stage 1 and disseminated disease into stage 3 (Table 1). The assessment of clinical stage for patients was done at time of admission (Day 0) and 24 h later (Day 1). (Table 2). Risk factors associated leading to cutaneous involvement in our sinonasal mucormycosis patients were detailed (Table 3). Out of 21 patients, 14 cases (66.6%) had a history of covid-19 infection. 5 of these patients had history of steroid intake (23.8%). 6 patients gave history of ICU stay (28.47%). All of these patients had deranged blood sugar levels and glycated haemoglobin (100%). Out of these only 7 patients were taking treatment for diabetes mellitus.
Liposomal amphotericin B was started with a dose of 5 mg/kg body weight (10 mg/kg body weight) in case of patients with cerebral involvement. Each vial contained 50 mg and was diluted in 5% or 10% dextrose and given slowly over 4–6 h. Renal functions and electrolytes were monitored. After resolution of signs of disease progression, treatment was stepped down to oral Posaconazole (300 mg delayed release tablets twice a day for 1 day followed by 300 mg daily) or Isavuconazole (200 mg 1 tablet 3 times daily for 2 days followed by 200 mg daily). Conventional Amphotericin B (deoxy cholate) in the dose 1-1.5 mg/kg was used if liposomal form was not available and renal functions and serum electrolytes of patients were within normal limits. Posaconazole was used in patients with renal dysfunction not tolerating amphotericin B.
Surgical debridement varied from endoscopic sinus surgery with removal of cutaneous necrotic tissue, total or partial maxillectomy and partial or complete orbital clearance. Daily dressing was done. Reconstruction of the defect was done by burns and plastic surgery department after a few months. Three patients could not be taken up for surgery as they were critical at the time of admission. Five patients expired in the early post-operative period. Our study observed a very high mortality rate of 38% (8 out of 21) in secondary cutaneous mucormycosis. Patients with higher stages of initial presentation had poor prognosis (Table 4). Mortality rate was highest in stage III with loss of 7 out of 14 patients (50%).
Two patients were not included in the study as even though they had initial similar presentation but histopathologically, one had features suggestive of actinomycosis and other patient had bacterial necrotising fasciitis.
Discussionl
Mucormycosis can lead to necrotic cutaneous lesions in immunocompromised patients, but other pathogens, such as Aspergillus, Fusarium, Pseudallescheria and Scedosporium species have to be kept in differential diagnosis [5]. Pseudomonas aeruginosa causing ecthyma gangrenosum may also produce a similar picture.
Primary form is usually due to direct inoculation. In secondary cutaneous mucormycosis, skin is infected most commonly after dissemination from rhinocerebral involvement. Predisposing factors include DM, with or without ketoacidosis, malignancies, transplantation, prolonged neutropenia, corticosteroids and other immunosuppressants and malnourishment [6]. The most affected areas of the skin are the arms and legs. Other locations include the scalp, face, thorax, back, abdomen, perineum, breast, neck and gluteal area.
Two third of our patients had history suggestive of Covid-19 infection. Many theories have been hypothesized in the literature to understand the pathophysiology of mucormycosis in Covid − 19 infection. In delta variant of Covid − 19 infection, unindicated high dose of steroids was found in many patients. Corticosteroids are known to impede migration, ingestion and phagolysosome fusion in macrophages. Steroids also increase blood glucose levels. Higher incidence of ketoacidosis has also been seen in patients on steroids. Few studies have reported a diminished growth of Rhizopus oryzae in iron deficient mediums, highlighting the importance of iron in the growth and metabolism of mucorales [7]. In SARS-CoV-2 infection, there is dysregulation of iron metabolism due to multiple factors. Higher incidence of ketoacidosis has been seen in Covid patients. Ketone bodies owing to its higher pH, inhibit the sequestration of iron by transferrin and ferritin and increase free serum iron. In response to Covid associated inflammation, a large amount of serum ferritin is released and also there is loss of its inner iron content soon after release. Hepcidin, an iron regulating hormone interacts with another transport protein ferroportin. SARS-CoV-2 glycoproteins mimic hepcidin leading to subsequent hyperferritinemia and ferroptosis. Covid virus also stimulates the synthesis of GRP78 receptor which is important for entry of virus into host cells. Moreover, upregulation of GRP78 also facilitates the fungal endocytosis through the same mechanism and enhances its pathogenicity and virulence [8, 9].
All our patients were diabetic proving deranged blood sugar to be a very important risk factor for rhinocerebral and secondary cutaneous mucormycosis. Very few reports have been published of cutaneous mucormycosis in immunocompetent individuals [10].
Clinical approach to diagnosis has a low sensitivity and specificity in early cases, it helps however in raising suspicion and prompting the initiation of laboratory testing. Histopathology, direct examination and culture remain essential tools, although the molecular methods are improving. Direct KOH microscopic examination (observing the presence of non-septate, hyaline hyphae with irregular branching at right angles mainly at the periphery of the lesion) helps in early detection [11]. Fungal culture performed in sabouraud and potato dextrose agar media is also positive in majority of cases. False positive cultures from non-sterile sites due to contamination have also been seen in a few cases. Histopathology with demonstration of angioinvasion, necrosis and non-septate or minimally septate hyphae is considered gold standard in diagnosis [12].
Cutaneous mucormycosis has a poor prognosis and requires prompt diagnosis and management with extensive surgical debridement, antifungal therapy and correction of the underlying metabolic or impaired immunological status.
The diagnostic sign that physicians usually associate with cutaneous mucormycosis namely necrotic eschar appears in late stages and although this sign is pathognomonic of the disease, it is important that one is aware that its absence does not exclude the diagnosis. In this era, patients with erythema and swelling may initially present in dermatology OPD and can be treated on lines of bacterial cellulitis- therefore, a high index of suspicion is necessary and patient should be sent for a diagnostic nasal endoscopy and radiological investigations if required. Our study also emphasizes on prompt management for these patients because of the rapidity of disease progression. We envisaged a staging system, with patients being categorised into different stages based on their cutaneous manifestation and sinus/extra sinus involvement. This staging helped us in prompt and effective management of our patients. For patients presenting in late stages, prognosis should be explained to the family members and a higher dose of liposomal Amphotericin B started immediately. Patients in stages I and II can be managed by endoscopic clearance along with systemic antifungals, but patients in stage III require early extensive open surgical debridement.
Our study illustrates the poor short-term prognosis of patients presenting with secondary cutaneous mucormycosis and highlights the efficient management of such cases by classifying them into sub-groups and early surgical intervention in such cases to reduce mortality due to this disease.
Conclusion
In this cohort, the prognosis of secondary cutaneous mucormycosis remained poor, especially in ICU patients, those accumulating numerous predisposing factors. Such patients presented in late stages of the disease and mortality rate was very high in such group. Appropriate antifungal therapy, repeated surgical debridement was critical for good outcome in patients with intermediate stages. In patients with comorbidities where surgery/ antifungal therapy was not possible, especially in the cases of disseminated infections, the short and medium-term prognosis was catastrophic.
References
Spellberg B, Edwards J Jr, Ibrahim A (2005) Novel perspectives on mucormycosis pathophysiology, presentation, and management. Clin Microbiol Rev 18:556–569
Paltauf A Mycosis mucorina: Ein Beitrag zur Kenntnis der menschilchen Fadenpiltzer-krankungen. Virchows Arch. Pathol. Anat. 1885, 102, 543–64
Anselmo-Lima WT, Lopes RP, Valera FC et al (2004) Invasive fungal rhinosinusitis in immunocompromised patients. Rhinology 42:141–144
Shrestha DB, Budhathoki P, Raut S, Adhikari S, Ghimire P, Thapaliya S et al (2021) New-onset diabetes in COVID-19 and clinical outcomes: a systematic review and meta-analysis. World J Virol 10:275–287
Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP (2012) Early clinical and Laboratory diagnosis of Invasive Pulmonary, Extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 54:55–60
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP (2012) Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54:23–34
Artis WM, Fountain JA, Delcher HK, Jones HE (1982) A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes 31:1109–1114
Sabirli R, Koseler A, Goren T, Turkcuer I, Kurt O (2021) High GRP78 levels in Covid-19 infection: a case-control study. Life Sci 265:118781
Jose A, Singh S, Roychoudhury A (2021) Current understanding in the pathophysiology of SARS-CoV-2-Associated Rhino-Orbito-Cerebral Mucormycosis: a Comprehensive Review. J Maxillofac Oral Surg 20:373–380
Xia ZK, Wang WL, Yang RY (2013) Slowly Progressive Cutaneous, Rhinofacial, and pulmonary mucormycosis caused by Mucor irregularis in an Immunocompetent Woman. Clin Infect Dis 56:993–995
Bonifaz A, Vázquez-González D, Tirado-Sánchez A, Ponce-Olivera RM (2012) Cutaneous zygomycosis. Clin Dermatol 30:413–419
Castrejon-Perez A, Welsh EC, Miranda I, Ocampo-Candiani J, Welsh O (2017) Cutaneous mucormycosis. An Bras Dermatol 92:304–311
Acknowledgements
NONE.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. Nikhil Arora, Dr. Ashiya Goel, Dr. Pratik Kumar, Dr Aditya Bhargava. The first draft of the manuscript was written by Dr. Nikhil Arora and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was done in accordance with the ethical standards of the institutional and/or national research committee or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent to participate and publish data
Informed and written consent was taken from the patients before enrolment in the study for their participation in the study and before the submission of the article and a written consent was also obtained for publishing their data in a journal article.
Conflict of interest
The authors declare “No conflict of interest”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arora, N., Goel, A., Kumar, P. et al. Secondary Cutaneous Mucormycosis – Retrospective Analysis From Tertiary Care Hospitall. Indian J Otolaryngol Head Neck Surg 75, 1712–1718 (2023). https://doi.org/10.1007/s12070-023-03707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-03707-3