Abstract
To assess the virucidal effect of povidone iodine (PVP-I) on severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) located in the nasopharynx and suitable dose-formulation for nasal application were the purpose of this clinical trial. This single-center, open-label randomized clinical trial with a 7-arm parallel-group design was conducted in Dhaka Medical College (DMC) Hospital. A total of 189 reverse transcription-polymerase chain reaction (RT-PCR)-confirmed SARS CoV-2 positive cases aged 12–90 years with symptoms was sequentially enrolled following randomization. Nasopharyngeal clearance of SARS-CoV-2 was tested against PVP-I nasal irrigation (NI) at diluted concentrations of 0.4%, 0.5% and 0.6%, and PVP-I nasal spray (NS) at diluted concentrations of 0.5% and 0.6%. All groups were compared to the corresponding controls (distilled water). Written informed consent was ensured before participation. All procedures were conducted in after ethical clearance from the Ethical Review Board and in accordance with the Declaration of Helsinki. Viral clearance in a repeat RT-PCR (qualitative) was the primary outcome, and occurrence of any adverse event following administration of testing drug was considered as the secondary outcome. Analysis was performed using SPSS (Version 26). All cases were randomized into seven groups and each group consists of 27-patient. Mean age of the cases 43.98 ± 12.67 years (SD). All strength of NI were effective in nasopharyngeal clearance compared to the control (0.4%, p = 0.006; 0.5%, p < 0.001; and 0.6%, p = 0.018). Similarly, all strength of the NS is also effective than control (0.5%, p = < 0.001; and 0.6%, p ≤ 0.001). Highest nasopharyngeal clearance was observed in patients using 0.5% NI (n = 25, 92.6%, p = 0.018). Nasal irritation was the single most adverse event recorded in this trial and found in two patients using 0.4%, and 0.6% PVP-I NI, respectively. Both PVP-I NS and NI are effective for nasopharyngeal clearance in-vivo. However, further community trials are needed to repurpose these solutions as preventive agents against SARS-CoV2.
Ethical clearance memo no ERC-DMC/ECC/2020/93.
Trial registration NCT Identifier number NCT04549376.
Similar content being viewed by others
Background
Coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread in 223 countries around the world, resulting in more than 125 million confirmed cases and more than 2.75 million deaths, as of March 27, 2021 [1]. This positive sensed, enveloped, single-stranded RNA virus resembles SARS-CoV and Middle East Respiratory Syndrome (MERS)-CoV which were responsible for the SARS 2003 and MERS 2012 epidemics, respectively [2, 3]. Inhalation of infected aerosol or respiratory droplet is the main mode of transmission of this virus which, upon entry, primarily colonize the nasopharynx [3, 4]. After binding and entry into the epithelial cells of respiratory tract, it starts replicating. A higher viral load is found in nasal swabs when compared to throat swabs [5]. Subsequently the virus migrates down the airways and enters alveolar epithelial cells resulting in cytokine storm as an anti-inflammatory response. This is thought to be the main cause of the catastrophic outcomes in COVID-19 patients. However, the viral load is highest with a high risk of pharyngeal shedding during the first week of symptom onset [3].
As nasopharynx and oropharynx act as a reservoir of the SARS-CoV-2, the application of virucidal agents to these surfaces may reduce viral burden and, thereby, might work as an effective preventive measure.
Povidone-iodine (PVP-I) is powerful bactericidal, fungicidal and virucidal agent that have been used for infection control and prevention for more than sixty years with adequate safety profile and very limited resistance report [6]. Interest in the use of PVP-I against coronaviruses first grew in response to SARS-CoV [7] and MERS-CoV [8] outbreaks in the past decade. Also, it had been found useful against some other viruses [9]. Homology of SARS CoV-2 virus with SARS CoV suggested that PVP-I based antiseptic preparations might be effective against it and were supported by several studies [10]. However, most of the studies were conducted in vitro and in a smaller scale. Nevertheless, several reports have also found effectiveness of PVP-I based antiseptic solutions in humans [11, 12]. Currently, there is no recommendation for suitable formulation (nasal spray/nasal irrigation) of PVP-I for nasal application as a prophylaxis measure. Therefore, the purpose of the randomized clinical trial was to-(1) investigate the virucidal activity of PVP-I nasal spray and nasal irrigation against SARS CoV-2 located on the nasopharynx among COVID-19-positive patients and (2) to find out the most suitable dilutions of PVP-I for nasal application.
Materials and Methods
Study Settings and Patients
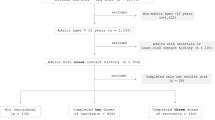
This single-center, open-label, parallel-group designed randomized clinical trial was conducted at the department of Otorhinolaryngology and Head Neck Surgery in collaboration with the Department of Virology and Department of Medicine in Dhaka Medical College Hospital (DMCH). The study included all COVID-19 cases who were RT-PCR positive in nasopharyngeal swabs within the previous 24 h and were symptomatic,between September 2020 and January 2021. Those who gave informed consent, were willing to participate, and accepted to be randomized to any assigned group were considered for inclusion. Patients with known sensitivity to PVP-I aqueous antiseptic solution or any of its listed excipients, who hadpreviously diagnosed thyroid disease,who had a history of chronic renal failure: stage ≥ 3 by estimated glomerular filtration rate (eGFR) using Modification of Diet in Renal Disease (MDRD) criteria,had acute renal failure (KDIGO ≥ stage 2: creatinine ≥ 2 times from the baseline), patients who were currently in invasive or noninvasive ventilation or were planned to be ventilated within the next 6 h were considered for exclusion. Moreover, lactating or pregnant women were also excluded from the trial. The authors followed the Extension of the Consolidated Standards of Reporting Trials (CONSORT) 2010 guideline [13]. The study protocol was published elsewhere [14].
Randomization of the Study Participants
The assignment to the study (intervention) or control (comparator) group were allocated in equal numbers through randomization using random number generation in Microsoft Excel by a statistician who was not involved in the trial. The allocation scheme was made by an independent statistician using a sealed envelope. The participants were allocated immediately after the eligibility assessment and consenting procedures. A total 189 confirmed COVID-19 cases were randomized and all patients completed the trial. As this was an open-label clinical trial, no blinding or masking was performed.
Intervention and Comparator
This RCT consist of seven arms:
Arm-1 (intervention group): received povidone iodine (PVP-I) nasal irrigation (NI) at a concentration of 0.4%
Arm-2 (intervention group): received PVP-I nasal irrigation at a concentration of 0.5%
Arm-3 (intervention group): received PVP-I nasal irrigation at a concentration of 0.6%.
Arm-4 (intervention group): received PVP-I nasal spray (NS) at a concentration of 0.5%.
Arm-5 (intervention group): received PVP-I nasal spray at a concentration of 0.6%.
Arm-6 (placebo comparator group): received distilled water through NI.
Arm-7 (Placebo comparator group): received distilled water through NS.
The intervention arms were compared to the placebo comparator arms. Other supportive and routine care were the same in all groups.
Formulation and Development of Test Drug
Main Outcomes
The primary outcome was the proportion of cases that remain COVID-19 positive following the intervention. The nasopharyngeal clearance was assessed by performing a repeat RT-PCR (qualitative). It was assessed immediately after the intervention and the duration ranged 1–15 min after the test drug administration. Any occurrence of adverse effects following the intervention were documented as a secondary outcome. Safety endpoint was the adverse event that required further investigation and care. Adverse events were counted in this study and was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Details of the grading are mentioned in supplementary file 1.
Methods of Data Collection
During admission, demographic and clinical details, including name, age/date of birth, gender, duration of clinical symptoms and hospitalization, vital statistics were recorded before randomization. A screening log were maintained including these essential details and the decision regarding participation made by the patient, attendant or legal guardian where appropriate. Ineligible and non-recruited patients were also cared by ward physicians following standard treatment guidelines. Data collection were performed by the trial physicians. A paper based semi-structured questionnaire was prepared and pretested before data collection. All potential participants were recorded in this database at first contact and assigned a study number. Study physicians recruited and randomized the patients with the help of statisticians. Allocation of the test drugs was performed by the principal investigator, and trial physicians confirmed the administration of PVP-I nasal irrigation (NI) at diluted concentrations of 0.4%, 0.5% and 0.6% and PVP-I nasal spray (NS) at diluted concentrations of 0.5% and 0.6%. Two group of the patients also received distilled water in the form of nasal spray (control NS or CNS) or nasal irrigation (control NI or CNI). All of the patients were monitored closely. Body temperature was measured every 6 h, and blood pressure, respiratory rate, and pulse rate were measured every hour. Urine output were measured in every 8 h up to 48 h. Drug-related side effects or any adverse events were observed in each group. If there is no reaction or unusual event, another RT-PCR sample were collected from the nasopharynx and sent for further testing. All clinical events were noted in case record form by trial physicians, including vital status at hospital discharge. Data entry were date/time stamped. Laboratory analysis data were collected using the same case record form.
Data Quality and Standards
Following collection, all data sets and collected record forms were checked. A formal clinical data management plan was prepared and agreed upon by the co-investigators and Data Monitoring Committee, and documented as a trial master file. Laboratory results were discussed regularly between the principal investigator (PI) and laboratory managers.
Ethics Statement
The study was approved by the ethical review committee of Dhaka Medical College Hospital (Memo no: ERC-DMC/ECC/2020/93). Then the trial protocol was registered at ClinicalTrials.gov on September 16, 2020 (NCT Identifier number: NCT04549376). Ethical guidelines of the Declaration of Helsinki and its succeeding conferences was followed throughout the study. Moreover, the study was conducted according to good clinical practice standards.
Statistical Analyses
Continuous data was expressed as mean with standard deviation and categorical data was expressed as frequency with percentage. Difference between the groups were estimated either by chi-square test (χ2) test or Fisher exact test or Independent sample-t test as appropriate. Logistic regression analysis was also performed to estimate the effect of the factors on the primary outcome. A p ≤ 0.05 was defined as statistically significant.
Results
A total of 189 confirmed COVID-19 cases were randomized into seven groups: 27 patients in each group. All of them completed the study. The average age of all participants was 43.9 ± 12.67 years (SD), and the highest proportion of them were aged between 31 and 40 years (n = 56, 29.6%). All intervention groups were statistically similar to their respective control groups in relation to age. Of all, 159 (84.1%) were male, and 30 (15.9%) were female. Among all groups, 0.6% NS group had statistically higher proportion of male (100%) compared to CNS (p = 0.01), but rest of groups didn’t differ significantly from respective controls in relation to sex. A total of 161 (85.2%) participants lived in urban area. All groups had statistically similar proportion of participants from Urban area except for 0.4%NI group which had significantly lower proportion of urban participants compared to CNI group (p = 0.036). Majority participants were employed (53.4%) and had a monthly family income between 20,001 to 40,000 BDT (46.6%). All groups were statistically similar by occupation and monthly family income. However, disease severity significantly differed between 0.5% NS and CNS (p < 0.001), and between 0.6%NS and CNS (p < 0.001) with the control group (CNS) having a significantly higher proportion of asymptomatic and mild patients. Diabetes was the most common comorbidity and was present in 64 (33.9%) patients. Hypertension, ischemic heart disease and bronchial asthma was present in 28 (14.8%), 9 (4.8%) and 2 (1.1%) patients, respectively. None of the intervention groups were significantly different from respective control in relation to presence of any individual comorbidity. But when presence of at least one comorbidity was considered, CNS group had significantly higher proportion of patients with comorbidities than 0.6%NS group (p = 0.029). The mean duration of disease was 1.86 ± 0.72 days which was statistically similar between intervention and control groups (Table 1).
We observed a statistically significant proportion of nasopharyngeal clearance with all strengths of PVP-I NI and PVP-I NS compared to the corresponding controls (p < 0.05). The only adverse event was nasal irritation recorded in two patients each in the 0.4% and 0.6% PVP-I NI groups which didn’t differ significantly from corresponding controls (p > 0.05). See Table 2 and Fig. 1 for details.
Table 3 describes the results of multivariable logistic regression analysis for factors associated with good outcome for each intervention groups separately. Good outcome was defined as RT-PCR negativity for COVID-19 after intervention indicating nasopharyngeal clearance. After adjusting for age, sex, severity, duration of disease and comorbidity all the interventions groups showed significantly higher odds of having a good outcome compared to corresponding controls. 0.4%NI, 0.5%NI and 0.6%NI groups were 9.27 times (95% confidence interval [CI] 2.06–41.75), 73.87 times (95%CI 8.26–660.14) and 136.19 times (95%CI 6.94–2671.21) more likely have good outcome compared to CNI, respectively. On a similar note, 0.5%NS and 0.6%NS groups were 56.96 times (95%CI 4.41–734.92) and 128.10 times (95%CI 6.79–2415.77) more likely to have good outcome compared to CNS, respectively. In these analyses, increasing age (Odds Ratio [OR] 1.12, 95%CI 1.02–1.22) and absence of comorbidity (OR 26.67, 95%CI 1.58–449.19) came as significant predictors of good outcome alongside 0.4%NI and 0.6%NI, respectively.
When all intervention and control groups were entered into the multivariable model 0.5%NI group had the highest chance (OR 141.883, 95%CI 15.92–1264.59) of having good outcome among all groups, when adjusted for age, sex, disease severity, duration of disease and comorbidity. All the adjusted factors became insignificant in this combined model. See Table 4 for details. A list of p-values determined for pairwise contrast of interventions across age, sex, severity, comorbidity is presented in supplementary table 1 for comparison.
Discussion
COVID-19 patients were found to carry high loads of SARS-CoV in their nasopharynx [15] and shed virus from there for a median 36 days after symptoms onset [16]. Hence, trials regarding use of nasal and oral antiseptic preparation to cut down viral loads were urged [17]. In vitro testing of PVP-I solution revealed a considerable reduction in SARS-CoV-2 viral titers [18], but the effect of using PVP-I solutions for nasopharyngeal clearance of the virus in humans is still unclear. Additionally, the strength of PVP-I solutions for this purpose were needed to be determined. Hence, we aimed to study the effectiveness and adverse events of different strength of nasal spray and nasal irrigation formulation of PVP-I in RT-PCR confirmed patients of COVID-19.
We found that all the preparations of PVP-I had significantly better nasopharyngeal clearance of SARS-CoV-2 among COVID-19 patients compared to respective controls. Our findings agree with the first report of in vivo PVP-I mouthwash application in COVID-19 patients [11]. However, that study reported the application of 15 ml 1.0% PVP-I mouthwash for 1 min rather than a nasal formulation in only 4 patients. Out of those 4 patients, 2 became RT-PCR negative for COVID-19 and 2 had a significant drop in viral loads. In contrast, Guenezan et al. [19] observed no effect of PVP-I 1% aqueous solution on nasopharyngeal viral loads compared to controls. Their intervention included 4 successive mouth wash with 25 mL of 1% aqueous PI solution followed by 2.5 ml nasal pulverization into each nostril. Hence, their method of application differed from that of ours. We used nasal irrigation and nasal spray formulation instead of nasal drop with an aim to reach the furthest wall of nasopharyngeal surface. As nasal epithelial cells show a high level of expression of ACE2 gene, and as SARS-CoV2 uses ACE2 for entry into cells [20] a thorough wash of nasopharyngeal epithelium is essential for adequate clearance of the virus.
Another important answer that our study intended to provide was the dilutions of PVP-I ought to be used as irrigation and spray. Anderson et al. [10]showed that PVP-I 1% gargle and mouth wash and 0.45% throat spray were able to achieve 99.99% virucidal activity against SARS-CoV-2 in vitro. A concentration as low as 0.23% were found effective against SARS-CoV2 in vitro [21]. On the other hand, Khan, Parab and Paranjape [22] showed that 0.5% povidone iodine solution can be safely used as gargle and nasal drops. Gluck et al. [23] used 2.2% and 4.4% PVP-I in liposomal dispersions in the form of nasal spray in a randomized controlled trial and found no noticeable adverse effects in mucosal appearance, olfactory function and ciliary motility. Therefore, we used 0.4%, 0.5% and 0.6% PVP-I nasal irrigation (NI) and 0.5% and 0.6% PVP-I nasal spray (NS) formulations in different arms and compared them with corresponding distilled water placebo formulations. Our analysis revealed that all the formulations were significantly better than respective controls in clearing the nasopharyngeal viral loads as evidenced by a negative RT-PCR result 1 to 15 min after the intervention. The baseline characteristics of 7-arms used in this trial were overall statistically comparable to each other and controls except for sex (between 0.6% NS and CNS), severity (between 0.5% NS and CNS, and 0.6% NS and CNS) and comorbidity (0.6% NS and CNS). To adjust these differences, we ran multivariate logistic regression, at first separately for each intervention and control pairs, and then incorporating all intervention and control groups. From the analysis every formulations of PVP-I were found to have significantly higher chance of nasopharyngeal clearance compared to control. Among all, 0.5% NI was the most effective PVP-I formation for getting a negative result in RT-PCR for COVID-19 after the intervention.
Additionally, the number of adverse events were very low in our study with only 4 (2.1%) cases of nasal irritation reported. Among them 2 (1.1%) were in 0.4% NI and another 2 (1.1%) were in 0.6%NI group. In contrast, Guenezan et al. [19] reported unpleasant nasal tingling and transient elevation of thyroid stimulating hormone after povidone iodine administration. Suppression of thyroid hormone production after an intervention with iodine-based antiseptic formulations could be a concern for the prescribing physician as iodine gets absorbed in blood. However, one study from Japan showed that daily intake of 1–3 mg would not produce any significant negative health effects, except a very low possibility of worsening symptoms in previously diagnosed cases of thyroid autoimmunity [24]. Therefore, it is safe to say that PVP-I nasal irrigation and nasal spray can be safely used for the rapid clearance of SARS-CoV-2 virus from the nasopharynx.
Nasal microenvironment has a central role in mediating entry and spread of SARS-CoV2 in humans [4]. Therefore, regular use of nasal antiseptics effective against the virus would theoretically prevent infection and also reduce spread from already infected individuals. However, from the single administration of either nasal irrigation or nasal spray in our study we are unable to advice a regular use. Nevertheless, our study showed PVP-I based nasal antiseptic solutions are safe to use. Hence, we recommend further trials with daily use regimens of PVP-I based nasal antiseptic solutions in a large sample of people to check its safety and efficacy in the prevention of COVID-19 infection.
Limitations of the Study
The limitation of our study was the small sample size in each arms, single center, lack of prolonged and repeated use regimens, and inability conduct more than onefollow-up RT-PCR testing over a longer period. We were also unable quantify the viral load before and after intervention. But, our study’s strength was the testing of different dilutions of PVP-I nasal solution and comparing those with distilled water controls in randomized multi-arm trial.
Conclusion
Povidone iodine can be applicable as nasal spray and nasal irrigation for nasopharyngeal clearance of SARS-Cov-2 virus located in nasopharynx in COVID-19 patients. Among the doses 0.5% in form of nasal irrigation are most efficient. Further community trial is warranted to test the suitability of the drug as a preventive agent against SARS-CoV-2 virus.
Availability of Data and Materials
The corresponding author has access to the all-trial information, and the data will be available on reasonable request (contact: dr.jahid61@gmail.com or arefin61dmc@gmail.com).
References
World Health Organization: WHO (2021) Coronavirus disease (COVID-19) pandemic, https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Cui J, Li F, Shi Z-L (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17:181–192
Hu B, Guo H, Zhou P et al (2021) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154
Gallo O, Locatello LG, Mazzoni A et al (2021) The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol 14:305–316
Zou L, Ruan F, Huang M et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1175–1177
Eggers M (2019) Infectious disease management and control with povidone iodine. Infect Dis Ther 8:581–593
Kariwa H, Fujii N, Takashima I (2006) Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 212:119–123
Eggers M, Eickmann M, Zorn J (2015) Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA). Infect Dis Ther 4:491–501
Sriwilaijaroen N, Wilairat P, Hiramatsu H et al (2009) Mechanisms of the action of povidone-iodine against human and avian influenza a viruses: its effects on hemagglutination and sialidase activities. Virol J 6:1–10
Anderson DE, Sivalingam V, Kang AEZ et al (2020) Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther 9:669–675
Lamas LM, Dios PD, Rodríguez MT et al (2020) Is povidone‐iodine mouthwash effective against SARS‐CoV‐2? First in vivo tests. Oral Diseases
Guenezan J, Garcia M, Strasters D et al (2021) Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19. JAMA Otolaryngol Neck Surg. https://doi.org/10.1001/jamaoto.2020.5490
Juszczak E, Altman DG, Hopewell S et al (2019) Reporting of multi-arm parallel-group randomized trials. JAMA 321:1610
Hasan MJ, Rumi SKNF, Banu SS et al (2021) Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: a structured summary of a study protocol for an open-label randomized clinical trial. Trials 22:2
Zou L, Ruan F, Huang M et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1177–1179
Mancuso P, Venturelli F, Vicentini M et al (2020) Temporal profile and determinants of viral shedding and of viral clearance confirmation on nasopharyngeal swabs from SARS-CoV-2-positive subjects: a population-based prospective cohort study in Reggio Emilia, Italy. BMJ Open. https://doi.org/10.1136/bmjopen-2020-040380
Stathis C, Victoria N, Loomis K et al (2021) Review of the use of nasal and oral antiseptics during a global pandemic. Future Microbiol 16:119–130
Hassandarvish P, Tiong V, Mohamed NA et al (2020) In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: implications for dental practice. Br Dent J. https://doi.org/10.1038/s41415-020-2402-0
Guenezan J, Garcia M, Strasters D et al (2021) Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19. JAMA Otolaryngol Neck Surg 9:1871
Sungnak W, Huang N, Bécavin C et al (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687
Kirk-Bayley J, Challacombe S, Sunkaraneni V et al (2020) The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may protect healthcare workers and reduce cross infection. SSRN Electron J. https://doi.org/10.2139/ssrn.3563092
Khan MM, Parab SR, Paranjape M (2020) Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am J Otolaryngol 41:102618
Gluck U, Martin U, Bosse B et al (2007) A clinical study on the tolerability of a liposomal povidone-iodine nasal spray: implications for further development. ORL 69:92–99
Nagata K, Takasu N, Akamine H et al (1998) Urinary iodine and thyroid antibodies in Okinawa, Yamagata, Hyogo, and Nagano, Japan: the differences in iodine intake do not affect thyroid antibody positivity. Endocr J 45:797–803
Acknowledgements
The authors acknowledge significant support from the Pi Research Consultancy Center, Dhaka, Bangladesh (www.pircc.org) in overall research activities. Also, thanks to the BRICM for funding and material support. The authors would like to thank all the clinical staff of the DMCH for their cordial assistance. Trial registration The trial protocol has been registered at ClinicalTrials.gov on September 16, 2020. NCT Identifier number: NCT04549376. (https://clinicaltrials.gov/ct2/show/NCT04549376)
Funding
The trial received research partial grant from BRICM (Bangladesh reference institute for chemical measurements).
Author information
Authors and Affiliations
Contributions
The MKA had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception of the research idea: MKA. Research design: MKA, AKMNU and MJh. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript: All authors. Statistical analysis: MASK, MKA and Mohammad Jahid hasan. Administrative, technical, or material support: AKMNU, MK and SSB. Supervision: MKA, SK Nurul Fattah Rumi, and Mohammad Jahid hasan. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics Approval
The RCT protocol was approved by the Ethical Review Committee of Dhaka Medical College (Memo no: ERC-DMC/ECC/2020/93) on 23 May 2020.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamal Arefin, M., Banu, S.S., Nasir Uddin, A.K.M. et al. Virucidal Effect of Povidone Iodine on SARS-CoV-2 in Nasopharynx: An Open-label Randomized Clinical Trial. Indian J Otolaryngol Head Neck Surg 74 (Suppl 2), 3283–3292 (2022). https://doi.org/10.1007/s12070-022-03106-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03106-0