Abstract
Extracorporeal membrane oxygenation for the purpose of intervening upon profound cardiovascular or pulmonary compromise has proven to be a worthy intervention. Technological advancements have allowed this mode of therapy to become more effective and widespread. Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is a commonly used strategy to help manage patients with pulmonary dysfunction refractory to traditional management methods. This review intends to focus upon common indications and the clinical considerations for the institution of VV-ECMO as well as some of its known complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a short-term treatment modality used for therapeutic intervention, during a period of respiratory or cardiac failure, not amenable to traditional methods of care. The first successful use of extracorporeal circulation was in 1954 by Dr. Gibbons and his team during an open heart bypass operation [1]. This precursor machine ultimately led to the current-day cardiopulmonary bypass machine. The first documented case of ECMO use in respiratory failure was in 1972 [2]. It was also during this time period that the first randomized study investigating ECMO for pulmonary dysfunction was published [3]. The high mortality rates in that study prevented widespread adoption of ECMO as a support strategy. While ECMO use was used in certain specific pediatric populations, it wasn’t until the H1N1 flu epidemic that it began regaining popularity in the adult populace [4]. One landmark trial in particular, the Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial, demonstrated improved outcomes with ECMO in respiratory failure patients [5]. This, in addition to advances in ECMO technologies and the development of more efficient circuits [6], has played a role in its current-day wider acceptance.
The purpose of this review is to present evidence for the use of veno-venous extracorporeal membrane oxygenation (VV-ECMO) for the treatment of respiratory compromise in addition to technical and practical aspects of its use.
Components
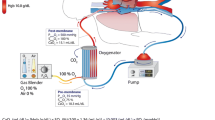
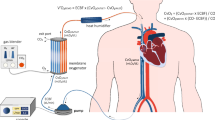
An ECMO circuit is comprised of a drainage and return cannula, blood pump, oxygenator, flow and pressure sensors, and heat exchanger [7]. The heat exchanger functions to cool or heat blood. Additionally, ECMO circuits also have venous and arterial points of access.
ECMO cannulation sites and cannula
ECMO can be instituted as veno-arterial (VA-ECMO) or veno-venous (VV-ECMO). Regardless of configuration, access is needed for both venous drainage and for blood return to the body, respectively. With VA-ECMO, when central cannulation is required, a thoracotomy or median sternotomy is performed [8]. This allows access to the central vessels, right atrium, or aorta. In peripheral VA-ECMO circuits, a venous cannula is placed in the femoral or jugular vein. Return is typically placed in the femoral or axillary artery [8]. For pulmonary issues, assuming adequate cardiac function, VV-ECMO is employed. This is routinely instituted percutaneously via the Seldinger technique and is often ultrasound guided [8]. Cannulas are wire reinforced to prevent bending. The correct placement of each cannula is confirmed via ultrasound, chest X-ray, fluoroscopy, or a combination of several techniques. In adult patients, the venous cannula is typically 50 to 70 cm with a diameter of 19 to 25 Fr [9]. In VV-ECMO, the drainage cannula is placed in the femoral vein and the return in the right atrium [9]. In our practice, as ECMO is often initiated under emergent conditions, we routinely first place both the drainage and return cannula in both the femoral veins, respectively. Thereafter, within 12–24 h, once the patient is stabilized, they are taken to the operating room for placement of a dual lumen cannula in the right internal jugular vein and both groin lines are removed. This type of access allows for drainage and return to take place via the same cannula and for patient movement following extubation.
Oxygenator
Typically, an oxygenator consists of receptacle comprised of two chambers which are divided by a semi-permeable membrane [10]. In one chamber, the patient’s blood will flow and in the other fresh gas flows. The semi-permeable membrane is termed the oxygenation membrane, where gas diffusion takes place, allowing for the oxygenation of the drained venous blood and removal of carbon dioxide [10]. This process is driven by gradients which allows for the oxygenation of venous return blood and the evacuation of carbon dioxide. The partial pressure of blood in the chamber, and thus the degree of oxygenated blood that will be returned to the patient, is determined by the amount of oxygen in the fresh gas flow which can be controlled via the ECMO circuit settings [10]. Conversely, the removal of carbon dioxide is driven by the gas flow rate, so increasing the flow will lead to more CO2 removal from the blood [10].
Blood pump
In an ECMO circuit, blood flow is driven by the blood pump. The popularity of roller pumps has given way to centrifugal pumps, a newer technology which uses a magnetic field to generate the force used to circulate blood [11]. This leads to decreased compression of blood, as compared to a roller pump, and thus a lower degree of inflammatory system activation [12]. Regardless of pump type, it is important that ECMO circuits have an alternate way for circuits to continue flowing in the case of patient transport or when a power failure occurs. This is often made possible via a battery and/or external hand crank.
Support for acute respiratory infection (CESAR trial H1N1, COVID)
It was during the H1N1 flu epidemic in 2009 that ECMO, or more specifically VV-ECMO, began gaining acceptance for the treatment of lung disease. The studies published before this time, however, were largely observational and sometimes contained conflicting results [13, 14]. As a result, many remained skeptical of this treatment modality. Further, it had not survived the rigors of a randomized study which would be required for more widespread acceptance. This void in the literature ultimately leads to the CESAR trial [5]. This randomized study, published in October 2009, demonstrated a significant improvement in death or severe disability at 6 months compared with conventional mechanical ventilation. Even with its increasing acceptance, some remained reluctant to adopt ECMO citing the percentage of patients in the CESAR study ECMO group who did not undergo ECMO (24%) and the absence of lung-protective ventilation in the control group. Another more recent trial performed in 2017, the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial [15], showed that although there was no difference in the 60-day mortality primary outcome, a secondary outcome of death or treatment failure (which was defined as death or crossover to ECMO in the control group) was significantly improved in the ECMO cohort. Crossover to emergency ECMO in the control group was 28%. Moreover, the EOLIA study protocol included the use of lung protective strategies, prone positioning, as well as neuromuscular blocking agents before and after study enrollment.
The initial wave of the coronavirus disease 2019 (COVID‐19) pandemic saw an intensive care unit admission rate of 21%, with 69% of these critical care admissions ultimately requiring mechanical ventilation [16]. Initial published data revealed high mortality rates associated with the use of ECMO in the COVID population and some providers deemed there was not enough evidence to support its use in this group of patients [17, 18]. In 2020, however, the Extracorporeal Life Support Organization (ELSO) registry published a cohort study of 1093 patients aged > 16 years who underwent VV-ECMO for respiratory insufficiency secondary to COVID-19. It was found that the estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 37.4% [19].
This mortality rate is consistent with that of which was seen in the CESAR study and EOLIA study, at 37% and 35%, respectively. With these data in mind, ECMO became a consistent tool in the armamentarium of those treating patients suffering from the acute pulmonary effects of COVID-19. Table 1 summarizes the inclusion and exclusion criteria suggested by the ELSO, ECMOnet, CESAR, and EOLIA studies.
Support for post-trauma (non-infectious) ARDS
The incidence of severe trauma leading to acute respiratory distress syndrome (ARDS) is roughly 10% [22]. This most commonly occurs after mechanisms including blunt thoracic trauma accompanied by severe pulmonary contusion, hypovolemic shock prompting massive transfusion, and flail chest [23].
The first described use of ECMO in the post-traumatic patient was published in 1972 [2].
Trauma patients are typically younger and healthier than non-trauma patients developing respiratory failure. These baseline characteristics are partially responsible for a favorable prognosis in trauma-related ARDS. Overall, although it is difficult to make direct comparisons, the data suggests that post-trauma VV-ECMO survival rates are non-inferior to those of adult patients, ranging from 50 to 79% [22, 24]. One study sought to determine if VV-ECMO improved survival in trauma patients suffering from acute hypoxemic respiratory failure and demonstrated that it was independently associated with improved survival. It was also seen that blood transfusions increased, and more bleeding complications were noted as a result [25].
Bridge to transplant
Due to supply and demand mismatches, organ availability continues to be a problem in lung transplantation. This is associated with increased wait-list times and the reported 10–20% mortality rate for patients while waiting [26]. Moreover, mechanical ventilation has been shown to be an inadequate bridging primary strategy [27]. The first reported case of ECMO as a bridge to lung transplant was in 1977 in a patient with post-traumatic respiratory failure who subsequently underwent bilateral lung transplantation [28]. The International Society of Heart and Lung Transplantation recommends ECMO as a bridge to transplant in young patients, without multiorgan dysfunction, with good rehabilitation potential. Patient selection in these circumstances is critical. In recent literature, the median duration of an ECMO bridge is 2–17 days [29, 30]. ECMO-associated bleeding is the most frequently reported complication [31,32,33]. Outcomes, however, have been shown to be good, with survival to lung transplantation ranging from 56 to 89% [31, 33, 34]. These survival findings are favorable given that patients in this population, often presenting in extremis, would likely expire prior to transplantation without the benefit of this treatment modality.
Support for post-transplant primary graft dysfunction (PGD)
PGD is defined by the International Society of Heart and Lung Transplantation (ISHLT) as a decreased partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio and the presence of diffuse infiltrates on thoracic imaging without other identifiable cause [35]. This devastating clinical circumstance is thought to occur in 15–30% of all lung transplants (LTx) [36, 37]. ECMO has been shown to improve survival in post LTx patients with PGD and, as such, PGD is the most common indication for post-transplant ECMO [38]. In order to minimize the harmful effects of elevated airway pressures, or high oxygen concentrations, prompt ECMO initiation has been recommended in the setting of PGD when peak airway pressures reach 35 cm of H2O or when FiO2 surpasses 60% [39]. If the modality is deemed necessary, delaying ECMO initiation greater than 48 h has been associated with worse outcomes. [40]
Clinical considerations with VV-ECMO
Mechanical ventilation management
One of the primary advantages of VV-ECMO is the reduction of both ventilator intensity and dependence. Lung protection strategies including plateau pressures < 30 mmHg, and tidal volumes 6 ≤ cc/kg, should be followed so as to decrease the risk of known ventilator-associated complications [41]. Additionally, patients should be extubated as appropriate. If unable to extubate, early tracheostomy should be considered as this helps to wean sedation, improve oral care, and reduce the incidence of vocal cord damage as a result of trans laryngeal endotracheal intubation [42].
Acute kidney injury
The overall incidence of acute kidney injury (AKI) in patients on VA and VV-ECMO is reported at 26–85% [43]. This wide range results from patient baseline characteristics, clinical circumstances, and differences in AKI definition. An AKI is also more common in VA-ECMO than VV-ECMO (61% vs 46%) and is most often present on the day of cannulation [44, 45]. Regardless of ECMO configuration, the underlying mechanism for the disruption of kidney function is theorized to be related to the systemic inflammatory response, intravascular volume depletion, hypotension, tissue hypoperfusion, and hemolysis [46]. For those requiring renal replacement therapy, continuous renal replacement therapy (CRRT) is recommended [47]. Consensus as to when to initiate CRRT, early or late, has not been reached [47].
Bleeding and intracranial hemorrhage
The risk of any bleeding with ECMO is reported to be as high as 29% [48], with a 10% risk of major bleeding, and a 4–10% risk of intracranial hemorrhage [49, 50]. There are many postulated reasons as to why bleeding is thought to be an issue in ECMO patients. First, ECMO circuits confer an elevated risk of thromboembolism due to blood exposure to non-biologic circuit and the non-pulsatile blood flow. This can lead to clot formation within the ECMO circuit and ultimately prompt a complete exchange of ECMO circuit components in 10–16% of cases [51]. As a result, ELSO endorses, in its latest international guidelines, the practice of an unfractionated heparin protocol to minimize the risk of circuit thrombosis although they also maintain that there is currently a paucity of evidence to guide optimal anticoagulation management in adult ECMO patients [52]. Furthermore, the most recent ELSO guidelines also state that the tendency towards less (lower or no) anticoagulation is potentially safe and feasible. As this continues to be an evolving topic, at our institution, we currently adhere to prior ELSO recommendations [53] where patients usually receive an initial unfractionated heparin bolus of 50–100 units per kilogram at the time of cannulation with an infusion then continued during the ECMO course. This infusion is initiated at dose of 7.5–20 units/kg/h in adults and titrated to maintain an activated partial thromboplastin time (PTT) of 60–90 s. This lab is typically drawn every 6 h. There are circumstances where anticoagulation is held, namely bleeding or profound coagulopathy as evidenced by lab values. At our institution, if the patient’s flow on ECMO is > 2 L per minute, then the withholding of anticoagulation is considered safe.
Accordingly, when anticoagulation is administered, the risk of bleeding increases. Furthermore, ECMO patients are commonly critically ill or in the postoperative period, both known conditions for increased risk for bleeding complications [54, 55]. Intracranial hemorrhage (ICH) is the most dreaded complication of extracorporeal life support. In some cohorts, it has been reported as the leading cause of death in VV-ECMO patients [56]. In the ECMO population, ICH is difficult to diagnose due to the sedation that is often required for these patients. Computed tomography imaging is the most commonly used tool to diagnose this complication but patient instability can delay or prevent this assessment [57]. The implications of this complication are devastating as the ELSO registry data reports that only 26% of VV-ECMO patients who develop ICH survive to discharge [58].
As VV-ECMO is a mechanism of support that can keep patients alive despite un-survivable injuries, determining when continued therapy is futile is a difficult process. This determination should be made as a collaborative discussion amongst surgeons, intensivists, and any other involved patient care team. If no meaningful recovery can be expected, providers should empathically state this to family, and then allow the family time to process this information, before recommending withdrawal of VV-ECMO therapy and transition to comfort care, if comfort care is thought possible [59].
Conclusion
VV-ECMO is a form of mechanical circulatory support that has been shown to be effective in temporarily managing patients with profound respiratory failure. It is important, therefore, that providers managing these patients are knowledgeable of its components, indications for use, patient populations demonstrated to benefit, and its clinical considerations, including common complications.
References
Gibbon JH Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–85; passim.
Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome) Use of the Bramson membrane lung. N Eng J Med. 1972;286:629–34. https://doi.org/10.1056/NEJM197203232861204.
Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6.
Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30. https://doi.org/10.1186/cc12512.
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63. https://doi.org/10.1016/S0140-6736(09)61069-2.
Betit P. Technical advances in the field of ECMO. Respir Care. 2018;63:1162–73. https://doi.org/10.4187/respcare.06320.
Tramm R, Ilic D, Davies AR, Pellegrino VA, Romero L, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2015;1:CD010381. https://doi.org/10.1002/14651858.CD010381.pub2.
Jayaraman AL, Cormican D, Shah P, Ramakrishna H. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: techniques, limitations, and special considerations. Ann Card Anaesth. 2017;20:S11–8. https://doi.org/10.4103/0971-9784.197791.
Squiers JJ, Lima B, DiMaio JM. Contemporary extracorporeal membrane oxygenation therapy in adults: fundamental principles and systematic review of the evidence. J Thorac Cardiovasc Surg. 2016;152:20–32. https://doi.org/10.1016/j.jtcvs.2016.02.067.
Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–14. https://doi.org/10.1056/NEJMct1103720.
Byrnes J, McKamie W, Swearingen C, et al. Hemolysis during cardiac extracorporeal membrane oxygenation: a case-control comparison of roller pumps and centrifugal pumps in a pediatric population. ASAIO J. 2011;57:456–61. https://doi.org/10.1097/MAT.0b013e31822e2475.
Johnson KN, Carr B, Mychaliska GB, Hirschl RB, Gadepalli SK. Switching to centrifugal pumps may decrease hemolysis rates among pediatric ECMO patients. Perfusion. 2022;37:123–7. https://doi.org/10.1177/0267659120982572.
Combes A, Luyt C-E, Nieszkowska A, Trouillet J-L, Gibert C, Chastre J. Is tracheostomy associated with better outcomes for patients requiring long-term mechanical ventilation? Crit Care Med. 2007;35:802–7. https://doi.org/10.1097/01.ccm.0000256721.60517.B1.
Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57. https://doi.org/10.1007/s00134-011-2301-6.
Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75. https://doi.org/10.1056/NEJMoa1800385.
Chang R, Elhusseiny KM, Yeh Y-C, Sun W-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-a systematic review and meta-analysis. PLoS ONE. 2021;16:e0246318. https://doi.org/10.1371/journal.pone.0246318.
Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–9. https://doi.org/10.1016/j.hrtlng.2020.03.012.
Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–8. https://doi.org/10.1016/j.jcrc.2020.03.011.
Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–8. https://doi.org/10.1016/s0140-6736(20)32008-0.
ELSO guidelines. Extracorporeal life support organization, version 1.4, August 2017, Ann Arbor, USA; available at: https://www.elso.org/resources/guidelines.aspx.
Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275–81. https://doi.org/10.1007/s00134-012-2747-1.
Bedeir K, Seethala R, Kelly E. Extracorporeal life support in trauma: worth the risks? A systematic review of published series. J Trauma Acute Care Surg. 2017;82:400–6. https://doi.org/10.1097/TA.0000000000001292.
Wu MY, Lin PJ, Tseng YH, Kao KC, Hsiao HL, Huang CC. Venovenous extracorporeal life support for posttraumatic respiratory distress syndrome in adults: the risk of major hemorrhages. Scand J Trauma Resusc Emerg Med. 2014;22:56. https://doi.org/10.1186/s13049-014-0056-0.
Jacobs JV, Hooft NM, Robinson BR, et al. The use of extracorporeal membrane oxygenation in blunt thoracic trauma: a study of the Extracorporeal Life Support Organization database. J Trauma Acute Care Surg. 2015;79:1049–53. https://doi.org/10.1097/TA.0000000000000790.
Swol J, Brodie D, Napolitano L, et al. Indications and outcomes of extracorporeal life support in trauma patients. J Trauma Acute Care Surg. 2018;84:831–7. https://doi.org/10.1097/TA.0000000000001895.
Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant. 2014;14:139–65. https://doi.org/10.1111/ajt.12584.
Elizur A, Sweet SC, Huddleston CB, et al. Pre-transplant mechanical ventilation increases short-term morbidity and mortality in pediatric patients with cystic fibrosis. J Heart Lung Transplant. 2007;26:127–31. https://doi.org/10.1016/j.healun.2006.11.597.
Veith FJ. Lung transplantation. Transplant Proc. 1977;9:203–8.
Todd EM, Biswas Roy S, Hashimi AS, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a single-center experience in the present era. J Thorac Cardiovasc Surg. 2017;154:1798–809. https://doi.org/10.1016/j.jtcvs.2017.06.063.
Yeo HJ, Lee S, Yoon SH, et al. Extracorporeal life support as a bridge to lung transplantation in patients with acute respiratory failure. Transplant Proc. 2017;49:1430–5. https://doi.org/10.1016/j.transproceed.2017.02.064.
Biscotti M, Gannon WD, Agerstrand C, et al. Awake extracorporeal membrane oxygenation as bridge to lung transplantation: a 9-year experience. Ann Thorac Surg. 2017;104:412–9. https://doi.org/10.1016/j.athoracsur.2016.11.056.
Hakim AH, Ahmad U, McCurry KR, et al. Contemporary outcomes of extracorporeal membrane oxygenation used as bridge to lung transplantation. Ann Thorac Surg. 2018;106:192–8. https://doi.org/10.1016/j.athoracsur.2018.02.036.
Hoetzenecker K, Donahoe L, Yeung JC, et al. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg. 2018;155:1316-1328.e1. https://doi.org/10.1016/j.jtcvs.2017.09.161.
Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg. 2012;144:716–21. https://doi.org/10.1016/j.jtcvs.2012.05.040.
Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, Part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1097–103. https://doi.org/10.1016/j.healun.2017.07.021.
Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–34. https://doi.org/10.1164/rccm.201210-1865OC.
Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS ONE. 2014;9:e92773. https://doi.org/10.1371/journal.pone.0092773.
Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93:366–71. https://doi.org/10.1016/j.athoracsur.2011.05.017.
Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis. 2014;6:1070–9. https://doi.org/10.3978/j.issn.2072-1439.2014.06.04.
Marasco SF, Vale M, Preovolos A, et al. Institution of extracorporeal membrane oxygenation late after lung transplantation - a futile exercise? Clin Transplant. 2012;26:E71–7. https://doi.org/10.1111/j.1399-0012.2011.01562.x.
Klompas M. Complications of mechanical ventilation — the CDC’s new surveillance paradigm. N Engl J Med. 2013;368:1472–5. https://doi.org/10.1056/NEJMp1300633.
Filice G, Patel P, Kata P, et al. An overview of outcomes associated with early versus late tracheostomy from a national standpoint. Cureus. 2021;13:e16325. https://doi.org/10.7759/cureus.16325.
Ostermann M, Lumlertgul N. Acute kidney injury in ECMO patients. Crit Care. 2021;25:313. https://doi.org/10.1186/s13054-021-03676-5.
Thongprayoon C, Cheungpasitporn W, Lertjitbanjong P, et al. Incidence and impact of acute kidney injury in patients receiving extracorporeal membrane oxygenation: a meta-analysis. J Clin Med. 2019;8:981. https://doi.org/10.3390/jcm8070981.
Delmas C, Zapetskaia T, Conil JM, et al. 3-month prognostic impact of severe acute renal failure under veno-venous ECMO support: importance of time of onset. J Crit Care. 2018;44:63–71. https://doi.org/10.1016/j.jcrc.2017.10.022.
Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, et al. Renal Replacement Therapy in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation. Clin J Am Soc Nephrol. 2012;7:1328–36. https://doi.org/10.2215/CJN.12731211.
Paek JH, Park S, Lee A, et al. Timing for initiation of sequential continuous renal replacement therapy in patients on extracorporeal membrane oxygenation. Kidney Res Clin Pract. 2018;37:239–47. https://doi.org/10.23876/j.krcp.2018.37.3.239.
Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017;7:51. https://doi.org/10.1186/s13613-017-0275-4.
Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85. https://doi.org/10.1164/rccm.201205-0815OC.
Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659–68. https://doi.org/10.1001/jama.2011.1471.
Doyle AJ, Hunt BJ. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med. 2018;5:352. https://doi.org/10.3389/fmed.2018.00352.
McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022;68:303–10. https://doi.org/10.1097/MAT.0000000000001652.
Laurance Lequier GA, Al-Ibrahim O, Bembea M, Brodie D, Brogan T, Buckvold S, Chicoine L, Conrad S, Cooper D, Dalton H, Frischer J, Harris B, Mazor R, Paden M, Rintoul N, Ryerson L, Spinella P, Teruya J, Winkler A, Wong T, Massicotte MP. ELSO Anticoagulation Guidelines. Guidelines. 2014. https://www.elso.org/ecmo-resources/guidelinesarchive.aspx
Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S-e277S. https://doi.org/10.1378/chest.11-2297.
Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–225. https://doi.org/10.1182/bloodadvances.2018022954.
Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. https://doi.org/10.1001/jama.2009.1535.
Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33:42–50. https://doi.org/10.1177/0267659118766435.
Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Crit Care Med. 2017;45:1389–97. https://doi.org/10.1097/CCM.0000000000002502.
Kasman DL. When is medical treatment futile? A guide for students, residents, and physicians. J Gen Intern Med. 2004;19:1053–6. https://doi.org/10.1111/j.1525-1497.2004.40134.x.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This document is a review of the current literature, without involvement of new human or animal subjects. As such, there is no need for EC approval.
Informed Consent
Not applicable as there is no patient identifying material.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights Statement
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ndubisi, N., van Berkel, V. Veno-venous extracorporeal membrane oxygenation for the treatment of respiratory compromise. Indian J Thorac Cardiovasc Surg 39 (Suppl 1), 18–24 (2023). https://doi.org/10.1007/s12055-022-01467-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01467-3