Abstract
Bronchial artery aneurysms (BAAs) are rare and are known to be associated with bronchiectasis. The presentation varies from incidental radiological finding to life-threatening hemoptysis. A diagnosis of BAA is an indication for intervention irrespective of its presentation. Despite interventional procedures being at the forefront of management, surgical procedures are being reserved for specific situations. Recently, video-assisted thoracoscopic surgery is an alternate for management of BAA. We, herein, present a case of multiple BAA with cystic bronchiectasis managed surgically with left lower lobectomy and localized descending thoracic aorta (DTA) replacement with plication of feeding arteries through left posterolateral thoracotomy approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bronchial artery aneurysms (BAAs) are rare entities which need early intervention to eliminate the risk of massive hemoptysis [1]. A definitive diagnosis and clear definition of anatomy of the aneurysm will decide the mode of management. BAA is usually managed by interventional procedures like bronchial artery embolization (BAE) or aortic stent [2,3,4]. Surgical options include clipping, ligating, or extirpation of the aneurysm through video-assisted thoracoscopic surgery (VATS) or open thoracotomy approach [5, 6].
Herein, we present a case of symptomatic multiple BAA managed surgically with left lower lobectomy and localized descending thoracic aorta (DTA) replacement.
Case report
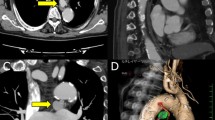
A 40-year-old gentleman, with a previous history of hypertension and mild Corona Virus Disease (COVID) illness, presented to us with complaints of breathlessness (modified Medical Research Council (mMRC) grade 2) and recurrent mild volume hemoptysis for 2 years. He was being treated in his hometown on a symptomatic basis. On presentation, he was hemodynamically stable with normal ambient saturation. Fine crepitations and wheeze were heard in the left basal zone on auscultation. Routine labs ruled out active infection and an echocardiogram revealed normal left ventricular function with normal sized ascending and descending aorta. However, a computerized tomography (CT) aortogram revealed a saccular aneurysm of the bronchial artery measuring 61 × 40 × 31 mm at T5-T6 of DTA along with three dilated tortuous collaterals at T4-5, T7, and T9 levels respectively (Fig. 1) along with predominant bronchiectatic changes in the left lower lobe (Fig. 2A, B). The pulmonary function test revealed a significant obstructive airway disease irreversible with bronchodilator therapy. No ventilation perfusion mismatch was noted on lung perfusion scan. After thorough multidisciplinary consultation and pre-anesthetic evaluation, he was taken up for surgery. A wide left posterolateral thoracotomy was done to gain adequate exposure along with harvesting of an intercostal muscle flap. The aneurysm was identified and defined only after meticulous adhesiolysis of the dense adhesions between the left lung chest wall and mediastinal pleura. The lesion was densely stuck to the lung parenchyma which mandated gentle handling. The diseased left lower lobe and part of lingula was subjected to a standard lobectomy with non-anatomical wedge resection, followed by buttressing of the bronchial stump with the previously raised intercostal muscle flap. A left heart bypass was established with left atrial (through the left superior pulmonary vein) and femoral cannulation at normothermia. The DTA was opened and the aneurysmal segment was excised, leaving behind the aneurysmal wall which was “stuck” to the lung parenchyma. Feeding arteries in the residual wall were oversewn with silk sutures. The DTA was replaced with a dacron woven graft of size 26 mm (Fig. 3). The total left heart bypass time was 90 min and “coming off” bypass was smooth. The whole procedure was uneventful and he was extubated after a few hours of mandatory ventilation. Postoperatively, the patient had residual pleural space issues which were managed with a suction device and aggressive chest physiotherapy. Histopathological examination of lung specimens confirmed the diagnosis of bronchiectasis and excised aorta segment with aneurysmal wall revealed chronic inflammatory changes.
A Computed tomography of aortogram mediastinal window, axial section showing large bronchial artery aneurysm of size 44 mm × 42 mm with a wide mouth in proximity with the aorta (marked with yellow arrow). B Three-dimensional reconstructed computed tomography aortogram showing multiple large bronchial artery aneurysms. Largest aneurysm marked with a white arrow, another aneurysm marked with a red arrow
Discussion
BAA is a rare vascular anomaly with incidence of < 1% [1]. Based on the etiology, they are classified as congenital, infective, inflammatory, or secondary to vasculitis, whereas, based on the location, they can be mediastinal or intra-pulmonary BAA [1]; it was the latter type in our patient. The clinical presentation can range from incidental radiological finding to massive hemoptysis secondary to aneurysm rupture. Our patient presented with dyspnea and hemoptysis with pre-existing cystic bronchiectasis while BAA was diagnosed on a CT aortogram. Although selective bronchial angiogram is the definitive diagnostic method for diagnosis, contrast-enhanced CT chest/aortogram has become a valid tool for BAA diagnosis, especially since it gives much more details about three-dimensional anatomy and adjacent structures [7]. The diagnosis of BAA by itself is an indication for intervention irrespective of the size or presentation. BAAs can be managed either surgically or through radiological intervention.
The choice of management depends on factors such as number, location, and origin of the aneurysm; length of the feeding arterial stump from the aorta; the overall anatomy; and the associated lesions.
BAE with coils, polyvinyl acetate, cyanoacrylate, or gelfoam has become the first line of management for BAA recently [2].
Our patient had multiple BAAs, with a maximum size of 61 × 40 × 31 mm, originating from the DTA at the T5-T6 level. They had extremely short stumps from the aorta and were in close proximity with the same (Fig. 1). The presence of cystic bronchiectasis in the left lower lobe also needed to be addressed.
The option of aortic stent graft with or without fibrin sealant/embolization of efferent feeding artery was discussed [3, 4]; however, we preferred surgical correction due to two reasons — the presence of multiple collaterals with a large aneurysm in close proximity to the aorta and associated bronchiectasis which warranted a parenchymal resection.
Moreover, recurrence after an interventional procedure is known to occur due to collateralization [1], which is further obviated by surgical correction.
Surgical correction in the form of extirpation of aneurysm or clipping/ligation of the feeding vessels through open or thoracoscopic approach has been reported often in the literature [5, 6]. In our case, the close proximity of the lesion to the DTA and dense adhesions to the healthier lung parenchyma ruled out the options of clipping/ligation of feeding vessels and complete extirpation, respectively. The aneurysmal sac was left open, after oversewing the origins of the feeding arteries, and we were forced to leave a residue of the wall of the aneurysmal sac on the lung. The DTA corresponding to BAA was replaced with a dacron graft of appropriate size, after establishing a left heart bypass.
Conclusion
BAAs are rare entities which can go completely unnoticed or present in dramatic circumstances. Irrespective of the presentation, BAA should be intervened as early as possible. CT studies with detailed delineation of the vascular anatomy are an acceptable alternative to bronchial angiography. The overall anatomy of the aneurysm plays a major role in deciding the mode of intervention. Although BAE has become the first-line management for straightforward cases, surgical management has a pivotal role in specific conditions. The replacement of DTA with closure of feeding arteries in a single stage is a good alternative to aortic stent graft or embolization of efferent vessels, provided, the necessary surgical expertise is available and patient safety is not compromised.
References
Tanaka K, Ihaya A, Horiuci T, et al. Giant mediastinal bronchial artery aneurysm mimicking benign esophageal tumor: a case report and review of 26 cases from literature. J Vasc Surg. 2003;38:1125–9.
Bak SH, Han H. Diagnosis of bronchial artery aneurysm by computed tomography: a case report. Radiol Case Rep. 2017;12:455–9.
San Norberto EM, Urbano García J, Montes JM, Vaquero C. Endovascular treatment of bronchial aneurysms. J Thorac Cardiovasc Surg. 2018;156:e109–17.
Matsumoto T, Uchida T, Ono T, et al. Bronchial artery aneurysm treated using aortic stent graft alone: a case report. Ann Vasc Dis. 2017;10:152–4.
Sanchez E, Alados P, Zurera L, et al. Bronchial artery aneurysm treated with aortic stent graft and fibrin sealant. Ann Thorac Surg. 2007;83:693–5.
Shiiya H, Suzuki Y, Yamazaki S, Kaga K. Thoracoscopic bronchial artery resection for multiple bronchial artery aneurysms. Ann Thorac Cardiovasc Surg. 2021;27:260–3.
Van den Eynde J, Arjomandi Rad A, Karasov I, et al. Open surgical correction of multiple bronchial artery aneurysms. J Card Surg. 2020;35:1657–9.
Funding
None.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Venkatesa Kumar Anakaputhur Rajan and Rohan Reddy Chinthareddy. All authors contributed in editing and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Statement of human and animal rights
Approval was obtained from the ethics committee (Narayana Health Academic Ethics Committee) – letter no. NHH/AEC-CL-2022–881 dated on 5 August 2022. The procedures used in the study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Waiver of consent was approved by the institutional ethics committee (NHAEC).
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajan, V.K.A., Chinthareddy, R.R., Mehra, S. et al. A novel surgical approach for multiple bronchial artery aneurysms. Indian J Thorac Cardiovasc Surg 39, 174–177 (2023). https://doi.org/10.1007/s12055-022-01436-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01436-w