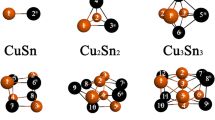
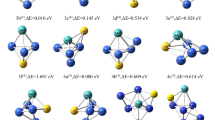
Abstract
Different decouplings of the Electron Propagator Theory (EPT) have been applied to many organic/inorganic compounds to get their vertical ionization potentials, while its potential in metal clusters is yet to be explored. In this study, we focus on assessing the reliability of this method for copper clusters, Cun (n = 1-13), as a case study. Furthermore, the wave function methods such as Hartree-Fock theory, Moller-Plesset perturbation theory, coupled-cluster theory, and long-range corrected density functional theory (LC-DFT) methods were also employed using the direct method (orbital eigenvalues) and indirect methods for comparison. The results show that outer-valance green function (OVGF) decoupling of the EPT methods gives a mean signed error (MSE) of 0.14 eV for the vertical ionization energies, which are lowest followed by partial third-order (P3) and modified partial third-order (P3+) methods. We presumed that the predicted vertical electron affinity using the EPT methods would be helpful for the experimentalists in the coming years. Therefore, electron propagator theory is reliable and should be explored extensively to get vertical ionization potentials and vertical electron affinities of other precious and non-precious metal clusters.
Graphical abstract
Different decouplings of the Electron Propagator Theory (EPT) have been applied to many organic/inorganic compounds to get their vertical ionization potentials, while its potential in metal clusters is yet to be explored. In this study, we focus on assessing the reliability of this method for copper clusters as a case study. It was found that the EPT is reliable, and this study encourages exploration of its untapped potential in other transition metal clusters.




Similar content being viewed by others
References
Luo Z, Castleman A W and Khanna S N 2016 Reactivity of metal clusters Chem. Rev. 36 14456
Chunyang D, Yinlong L, Danyang C, Mengtao Z, Jinjia L, Yang-Gang W, et al. 2020 Supported metal clusters: fabrication and application in heterogeneous catalysis ACS Catal. 10 11011
Herzing A A, Kiely C J, Carley A F, Landon P and Hutchings G J 2008 Identification of active gold nanoclusters on iron oxide supports for CO oxidation Science 321 1331
Qian L and Zheng G 2020 Recent advances of metal nanoclusters for aerobic oxidation Mater. Today Nano 11 100080
Yamamoto K, Imaoka T, Chun W J, Enoki O, Katoh H, Takenaga M and Sonoi A 2009 Size-specific activity of Pt clusters enhances oxygen reduction reaction Nat. Chem. 1 397
Tang Z, Wu W and Wang K 2018 Oxygen reduction reaction catalyzed by noble metal clusters Catalysts 8 65
Oliver-Meseguer J, Cabrero-Antonino J R, Dominguez I, Leyva-Perez A and Corma A 2012 Small gold clusters formed in solution give reaction turnover numbers of 10(7) at room temperature Science 338 1452
Vajda S, Pellin M J, Greeley J, Marshall C L, Curtiss L A, Ballentine G A, et al. 2009 Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane Nat. Mater. 8 213
Okrut A, Runnebaum R C, Ouyang X, Lu J, Aydin C, Hwang S-J, Zhang S Olatunji-Ojo O A, Durkin K A, Dixon D A, Gates B C and Katz A 2014 Selective molecular recognition by nanoscale environments in a supported iridium cluster catalyst Nat. Nanotechnol. 9 459
Qiao Z-A, Zhang P, Chai S-H, Chi M, Veith G M, Gallego N C, et al. 2014 Lab-in-a-shell: encapsulating metal clusters for size sieving catalysis J. Am. Chem. Soc. 136 11260
Corma A, Concepción P, Boronat M, Sabater M J, Navas J, Yacaman M J, et al. 2013 Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity Nat. Chem. 5 775
Koopmans TA 1933 Ordering of wave functions and eigen energies to the individual electrons of an atom Physica 1 104
Yang W, Zhang Y and Ayers P 2000 Degenerate ground states and a fractional number of electrons in density and reduced density matrix functional theory Phys. Rev. Lett. 84 5172
ížek J Cˇ 1966 On the correlation problem in atomic and molecular systems: Calculation of wavefunction components in ursell-type expansion using quantum-field theoretical methods J. Chem. Phys. 45 4256
Purvis G D III and Bartlett R J 1982 A full coupled-cluster singles and doubles model: the inclusion of disconnected triples J. Chem. Phys. 76 1910
Scuseria G E and Schaefer H F III 1989 Is coupled-cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration interaction (QCISD)? J. Chem. Phys. 90 3700
Scuseria G E, Janssen C L and Schaefer H F III 1988 An efficient reformulation of the closed-shell coupled-cluster single and double excitation (CCSD) equations J. Chem. Phys. 89 7382
Leszczynski J 2012 Handbook of Computational Chemistry (Dordrecht: Springer) p. 2
Szabo A and Ostlund N S 1989 Modern quantum chemistry: Introduction to advanced electronic structure theory (London: Dover Publications)
Froese Fischer C 1977 Hartree-Fock Method for Atoms. A Numerical Approach (New York: John Wiley and Sons)
Møller C and Plesset M S 1934 Note on an approximation treatment for many electron systems Phys. Rev. 46 618
Leininger T, Stoll H, Werner H-J and Savin A 1997 Combining long-range configuration interaction with short-range density functionals Chem. Phys. Lett. 275 151
Iikura H, Tsuneda T, Yanai T and Hirao K 2001 A long-range correction scheme for generalized-gradient-approximation exchange functionals J. Chem. Phys. 115 3540
Tawada Y, Tsuneda T, Yanagisawa S, Yanai T and Hirao K 2004 A long range-corrected time-dependent density functional theory J. Chem. Phys. 120 8425
Yanai T, Tew D P and Handy N C 2004 A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP) Chem. Phys. Lett. 393 51
Song JW, Hirosawa T, Tsuneda T and Hirao K 2007 Long-range corrected density functional calculations of chemical reactions: redetermination of parameter J. Chem. Phys. 126 154105
Tsuneda T, Song J-W, Suzuki S and Hirao K 2010 On Koopmans’ theorem in density functional theory J. Chem. Phys. 133 174101
Tsai C-W, Su Y-C, Li G-D and Chai J-D 2013 Assessment of density functional methods with correct asymptotic behaviour Phys. Chem. Chem. Phys. 15 8352
Singh R K and Tsuneda T 2013 Reaction energetics on long-range corrected density functional theory: Diels-Alder reactions J. Comput. Chem. 34 379
Tsuneda T and Singh R K 2014 A reactivity index based on orbital energies J. Comput. Chem. 35 1093
Singh RK, Kunimatsu K, Miyatake K and Tsuneda T 2016 Experimental and theoretical study on infrared spectrum of hydrated Nafion membrane Macromolecules 49 6621
Tsuneda T, Singh R K and Nakata A 2017 On low-lying excited states of extended nanographenes J. Comput. Chem. 38 2020
Tsuneda T, Singh R K and Chattaraj P K 2018 Diagrams for comprehensive molecular orbital-based chemical reaction analyses: reactive orbital energy diagrams Phys. Chem. Chem. Phys. 20 14211
Singh R K and Taketsugu T 2018 Insights into the geometries, stabilities, electronic, reactivity descriptors, and magnetic properties of the bimetallic NimCun-m (m=1, 2, n=3-13) clusters: comparison with pure copper clusters J. Comput. Chem. 39 1878
Singh S, Taketsugu T and Singh R K 2021 Hydration, prediction of the pKa, and infrared spectroscopic study of sulfonated polybenzophenone (SPK) block-copolymer hydrocarbon membranes and comparisons with Nafion ACS Omega 6 32739
Ortiz J V 1997 The Electron propagator picture of molecular electronic structure In Computational Chemistry: Reviews of Current Trends J Leszczynski (Ed.) (Singapore: World Scientific) Vol. 2 pp. 1−61
von Niessen W, Schirmer J and Cederbaum L S 1984 Computational methods for the one-particle green’s function Comput. Phys. Rep. 1 57
Ortiz J V 1999 Toward an exact one-electron picture of chemical bonding Adv. Quant. Chem. 35 33
Linderberg J and Öhrn Y 2004 Propagators in Quantum Chemistry 2nd edn. (Hoboken, NJ: Wiley-Interscience)
Ortiz J V 2013 Electron propagator theory: an approach to prediction and interpretation in quantum chemistry Wires Comput. Mol. Sci. 3 123
Mishra M K and Medikeri M N 1996 Characterization of shape and auger resonances using the dilated one-electron propagator method Adv. Quant. Chem. 27 223
Dolgounitcheva O, Zakrzewski V G and Ortiz J V 2005 Electron propagator calculations show that alkyl substituents alter porphyrin ionization energies J. Am. Chem. Soc. 127 8240
Dolgounitcheva O, Zakrzewski V G and Ortiz J V 2005 Ab initio electron propagator calculations on the ionization energies of free base porphine, magnesium porphyrin, and zinc porphyrin J. Phys. Chem. A 109 11596
Zakjevskii V V, King S J, Dolgounitcheva O, Zakrzewski V G and Ortiz J V 2006 Base and phosphate electron detachment energies of deoxyribonucleotide anions J. Am. Chem. Soc. 128 13350
Zakjevskii V V, Dolgounitcheva O, Zakrzewski V G and Ortiz J V 2007 Electron propagator studies of vertical electron detachment energies and isomerism in purinic deoxyribonucleotides Int. J. Quant. Chem. 107 2266
Melin J, Singh R K, Mishra M K and Ortiz J V 2007 Tautomeric forms of azolide anions: vertical electron detachment energies and dyson orbitals J. Phys. Chem. A 111 13069
Singh R K and Mishra M K 2009 Investigation of ethynylfurans using the electron propagator theory J. Phys. Chem. A 113 14150
Singh R K, Ortiz J V and Mishra M K 2010 Tautomeric forms of adenine: vertical ionization energies and dyson orbitals Int. J. Quant. Chem. 110 1901
Zakrzewski V G, Dolgounitcheva O, Zakjevskii A V and Ortiz J V 2011 Ab initio electron propagator calculations on electron detachment energies of fullerenes, macrocyclic molecules, and nucleotide Fragments Adv. Quant. Chem. 62 105
Dutta J, Mandal S, Adhikari S, Spiering P, Meyer J and Somers M F 2021 Effect of surface temperature on quantum dynamics of H2 on Cu(111) using a chemically accurate potential energy surface J. Chem. Phys. 154 104103
Dutta J, Mandal S and Adhikari S 2022 Formulation of temperature dependent effective Hartree potential incorporating quadratic over linear molecular DOFs-surface modes couplings and its effect on quantum dynamics of D2 (v = 0, j = 0)/D2 (v = 0, j = 2) on Cu(111) metal surface Chem. Phys. 552 111371
Gaussian 16, Revision A.03, Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Petersson G A, Nakatsuji H, Li X, Caricato M, Marenich A V, Bloino J, Janesko B G, Gomperts R, Mennucci B, Hratchian H P, Ortiz J V, Izmaylov A F, Sonnenberg J L, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski V G, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery J A, Jr Peralta J E, Ogliaro F, Bearpark M J, Heyd J J, Brothers E N, Kudin K N, Staroverov V N, Keith T A, Kobayashi R, Normand J, Raghavachari K, Rendell A P, Burant J C, Iyengar S S, Tomasi J, Cossi M, Millam J M, Klene M, Adamo C, Cammi R, Ochterski J W, Martin R L, Morokuma K, Farkas O, Foresman J B, Fox D J Gaussian, Inc., Wallingford CT, 2016.
Weigend F and Ahlrichs R 2005 Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy Phys. Chem. Chem. Phys. 7 3297
Knickelbein M B 1992 Electronic shell structure in the ionization potentials of copper clusters Chem. Phys. Lett. 192 129
Ingolfsson O, Busolt U and Sugawara K 2000 Energy-resolved collision-induced dissociation of Cu+n (n=2–9): stability and fragmentation pathways J. Chem. Phys. 112 4613
Acknowledgements
This research was supported by the University Grants Commission (UGC)-BSR Research Start-Up Grant, No. F. 30-347/2019(BSR). Anjani Nandan Pandey, a Ph.D. scholar, is grateful to Jai Prakash University, Chapra, for providing the facilities.
Funding
Funding was supported by UGC-DAE Consortium for Scientific Research, University Grants Commission, 30-347/2019(BSR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandey, A.N., Taketsugu, T. & Singh, R.K. Theoretical investigation of copper clusters using the electron propagator theory. J Chem Sci 135, 27 (2023). https://doi.org/10.1007/s12039-023-02146-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02146-5