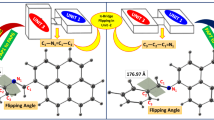
Abstract
Time-dependent density functional theory implementing optimally-tuned range-separated hybrid combined with an appropriate solvent model is used to understand the pH-driven changes in the experimentally observed photophysical properties of a novel \(\pi \)-conjugated aryl hydrazone-derivative of dicyanomethylene-dihydro-trimethylfuran-carbonitrile (termed as DCDHF-H) dye in the polar solvent. Calculations reveal similar planar structures for the solvated ground and excited states of the DCDHF-H dye at low solution pH, leading to high fluorescence quantum yield. On the other hand, photoexcitation and subsequent charge-polarization triggered very fast excited-state nuclear relaxation of the deprotonated dye at high solution pH, producing a twisted structure from the planar ground state, which explains the observed fluorescence quenching. Only a small charge transfer is found at the solvated Franck-Condon and relaxed emissive state geometries. Importantly, the large electron-phonon couplings associated with the soft vibrational modes are found to open up nonradiative deactivation channels for the deprotonated form of the dye. Additionally, the presence of a low-lying triplet with remarkably high SOC between the \({S}_{1}\) and \({T}_{2}\) also opens up active nonradiative ISC channel only for the deprotonated dye, supporting the observed fluorescence switching off. These results are useful to understand the reported experimental observations better and provide valuable insights into designing small \(\pi \)-conjugated organic molecules for sensing and imaging applications.
Graphical Abstract
Rationales for the pH-triggered fluorescence switching in an aryl hydrazone-based dye in a polar solvent are provided using reliable quantum-chemical calculations.




Similar content being viewed by others
References
Han H-H, Tian H, Zang Y, Sedgwick A C, Li J, Sessler J L, et al. 2021 Small-molecule fluorescence-based probes for interrogating major organ diseases Chem. Soc. Rev. 50 9391
Yin J, Huang L, Wu L, Li J, James T D and Lin W 2021 Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions Chem. Soc. Rev. 50 12098
Wu D, Sedgwick A C, Gunnlaugsson T, Akkaya E U, Yoon J and James T D 2017 Fluorescent chemosensors: the past, present and future Chem. Soc. Rev. 46 7105
Pham T C, Nguyen V N, Choi Y, Lee S and Yoon J 2021 Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy Chem. Rev. 121 13454
Wu L, Huang C, Emery B P, Sedgwick A C, Bull S D, He X-P, et al. 2020 Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents Chem. Soc. Rev. 49 5110
Liu X and Chang Y-T 2022 Fluorescent probe strategy for live cell distinction Chem. Soc. Rev. 51 1573
Yang M, Fan J, Du J and Peng X 2020 Small-molecule fluorescent probes for imaging gaseous signaling molecules: current progress and future implications Chem. Sci. 11 5127
Xiao H, Li P and Tang B 2021 Small Molecular Fluorescent Probes for Imaging of Viscosity in Living Biosystems Chem. Eur. J. 27 6880
Wen Y, Jing N, Huo F and Yin C 2021 Recent progress of organic small molecule-based fluorescent probes for intracellular pH sensing Analyst 146 7450
Ludwanowski S, Samanta A, Loescher S, Barner-Kowollik C and Walther A 2020 A Modular Fluorescent Probe for Viscosity and Polarity Sensing in DNA Hybrid Mesostructures Adv. Sci. 8 2003740
Ahmed R and Manna A K 2021 Origins of Large Stokes Shifts in a Pyrene–Styrene-Based Push-Pull Organic Molecular Dyad in Polar Solvents and Large Electron Mobility in the Crystalline State: A Theoretical Perspective J. Phys. Chem. C 126 423
Sasaki S, Drummen G P C and Konishi G 2016 Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry J. Mater. Chem. C 4 2731
Doroshenko A O and Pivovarenko V G 2003 Fluorescence quenching of the ketocyanine dyes in polar solvents: anti-TICT behavior J. Photochem. Photobiol. A: Chem. 156 55
Grabowski Z R, Rotkiewicz K and Rettig W 2003 Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures Chem. Rev. 103 3899
Kise K, Hong Y, Fukui N, Shimizu D, Kim D and Osuka A 2018 Diarylamine-Fused Subporphyrins: Proof of Twisted Intramolecular Charge Transfer (TICT) Mechanism Chem. Eur. J. 24 8306
Steinegger A, Wolfbeis O S and Borisov S M 2020 Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications Chem. Rev. 120 12357
Di Costanzo L and Panunzi B 2021 Visual pH Sensors: From a Chemical Perspective to New Bioengineered Materials Molecules 26 2952
Wang S and Kim S-H 2009 Photophysical and electrochemical properties of D–π–A type solvatofluorchromic isophorone dye for pH molecular switch Curr. Appl. Phys. 9 783
Liu X, Wang N, Lv L and Cai L 2011 Theoretical investigation on proton-induced intramolecular charge transfer of a D-π-A dye for a pH molecular switch Comput. Theor. Chem. 978 29
Sit H-Y, Deng J-R, Chan W-C, Ko BC-B and Wong M-K 2022 Quinolizinium-based tunable pH fluorescent probes for imaging in live cells Dyes Pigm. 205 110541
Khattab T A, Tiu B D B, Adas S, Bunge S D and Advincula R C 2016 pH triggered smart organogel from DCDHF-hydrazone molecular switch Dyes Pigm. 130 327
Chai J and Head-Gordon M 2008 Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections Phys. Chem. Chem. Phys. 10 6615
Scalmani G and Frisch M J 2010 Continuous surface charge polarizable continuum models of solvation. I. General formalism J. Chem. Phys. 132 144110
Stein T, Kronik L and Baer R 2009 Prediction of charge-transfer excitations in coumarin-based dyes using a range-separated functional tuned from first principles J. Chem. Phys. 131 244119
Kronik L, Stein T, Refaely-Abramson S and Baer R 2012 Excitation Gaps of Finite-Sized Systems from Optimally Tuned Range-Separated Hybrid Functionals J. Chem. Theory Comput. 8 1515
Phillips H, Zheng Z, Geva E and Dunietz B D 2014 Orbital gap predictions for rational designs of organic photovoltaic materials Org. Elec. 15 1509
Manna A K, Lee M H, McMahon K L and Dunietz B D 2015 Calculating High Energy Charge Transfer States using Optimally Tuned Range-Separated Hybrid Functionals J. Chem. Theory Comput. 11 1110
Ahmed R and Manna A K 2021 Theoretical insights on tunable optoelectronics and charge mobilities in cyano-perylenediimides: interplays between –CN numbers and positions Phys. Chem. Chem. Phys. 23 14687
Ahmed R and Manna A K 2020 Molecular-scale engineering of the charge-transfer excited states in non-covalently bound Zn–porphyrin and carbon fullerene based donor–acceptor complex Phys. Chem. Chem. Phys. 22 14822
Körzdörfer T and Brèdas J-L 2014 Organic Electronic Materials: Recent Advances in the DFT Description of the Ground and Excited States Using Tuned Range-Separated Hybrid Functionals Acc. Chem. Res. 47 3284
Janak J 1978 Proof that ∂E/∂ni = εi in density-functional theory Phys. Rev. B 18 7165
Strickler S and Berg R A 1962 Relationship between Absorption Intensity and Fluorescence Lifetime of Molecules J. Chem. Phys. 37 814
Sarkar S, Protasiewicz J D and Dunietz B D 2018 Controlling the Emissive Activity in Heterocyclic Systems Bearing C=P Bonds J. Phys. Chem. Lett. 9 3567
Bhandari S, Sarkar S, Schubert A, Yamada A, Payne J, Ptaszek M, et al. 2021 Intersystem Crossing in Tetrapyrrolic Macrocycles A First-Principles Analysis J. Phys. Chem. C 125 13493
Ahmed R and Manna A K 2022 Understanding High Fluorescence Quantum Yield and Simultaneous Large Stokes Shift in Phenyl Bridged Donor-π-Acceptor Dyads with Varied Bridge Lengths in Polar Solvents J. Phys. Chem. A 126 4221
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, et al. 2016 Gaussian 16, rev. B.01 (Gaussian Inc.: Wallingford, CT)
Shao Y, Gan Z, Epifanovsky E, Gilbert A T B, Wormit M and Kussmann J et al. 2015 Advances in Molecular Quantum Chemistry Contained in the Q-Chem 4 Program Package Mol. Phys. 113 184
Reimers J R 2001 A practical method for the use of curvilinear coordinates in calculations of normal-mode-projected displacements and Duschinsky rotation matrices for large molecules J. Chem. Phys. 115 9103
Sasikumar D, John A T, Sunny J and Hariharan M 2020 Access to the triplet excited states of organic chromophores Chem. Soc. Rev. 49 6122
Ahmed R and Manna A K 2022 Origins of Molecular-Twist-Triggered Intersystem Crossing in Functional Perylenediimides: Singlet-Triplet Gap versus Spin-Orbit Coupling J. Phys. Chem. A 126 6594
Ahmed R and Manna A K 2022 Tailoring intersystem crossing of perylenediimide through chalcogen-substitution at bay-position: A theoretical perspective J. Chem. Phys. 157 214301
Peach M J G, Williamson M J and Tozer D J 2011 Influence of Triplet Instabilities in TDDFT J. Chem. Theory Comput. 7 3578
Gao X, Bai S, Fazzi D, Niehaus T, Barbatti M and Thiel W 2017 Evaluation of Spin-Orbit Couplings with Linear-Response Time-Dependent Density Functional Methods J. Chem. Theory Comput. 13 515
Acknowledgments
We gratefully acknowledge IIT Tirupati for providing the infrastructure and generous research support. AKM thanks DST-SERB (Grant No.: ECR/2017/001903) and DST-Inspire (Grant No.: DST/Inspire/04/2016/000137), Govt. of India for providing research grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor S. P. Bhattacharyya on the occasion of his 75th birthday.
Special Issue on Interplay of Structure and Dynamics in Reaction Pathways, Chemical Reactivity and Biological Systems.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, R., Manna, A.K. Understanding pH Tailored Photophysical Properties of a \({\varvec{\pi}}\)-Conjugated Aryl Hydrazone-Derived Dye for Sensing Application. J Chem Sci 135, 9 (2023). https://doi.org/10.1007/s12039-022-02129-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-022-02129-y