Abstract
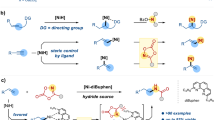
AlCl3 acts as a chlorinating agent for ynamides in the presence of stoichiometric amount of water in the environmentally benign solvent dimethylcarbonate, affording efficient access to (E)-α-chloroenamides via hydrochlorination, with water as a protic source. The role of water in the reaction was proven by deuterium labelling experiment. Epoxy-ynamides undergo iodochlorination in addition to the cleavage of the epoxy ring to afford (E/Z)-α-chloro-β-iodo-enamides. Regio- and stereochemical assignments for the products are based on X-ray crystallographic studies.
Graphic abstract
Chloroenamides are formed regio- and stereo-specifically via hydrochlorination of ynamides with AlCl3+H2O (1:1) in a dimethyl carbonate; aryl substituted epoxy ynamides afford (iodo)(chloro) enamides upon treatment with ICl.









Similar content being viewed by others
References
Selected recent reviews: (a) Dekorver K A, Li H, Lohse A G, Hayashi R, Lu Z, Zhang Y and Hsung R P 2010 Ynamides: A Modern Functional Group for the New Millennium Chem. Rev. 110 5064; (b) Evano G, Coste A and Jouvin K 2010 Ynamides: Versatile Tools in Organic Synthesis Angew. Chem., Int. Ed. 49 2840; (c) Wang X-N, Yeom H-S, Fang L-C, He S, Ma Z-X, Kedrowski B L and Hsung R P 2014 Ynamides in Ring Forming Transformations Acc. Chem. Res. 47 560; (d) Lu T and Hsung R P 2014 Novel ynamide structural analogues and their synthetic transformations Arkivoc i 127; (e) Evano G, Jouvin K and Coste A 2013 General Amination Reactions for the Synthesis of Ynamides Synthesis 45 0017; (f) Pan F, Shu C and Ye L-W 2016 Recent progress towards gold-catalyzed synthesis of N-containing tricyclic compounds based on ynamides Org. Biomol. Chem. 14 9456; (g) Prabagar B, Ghosh N and Sahoo A K 2017 Cyclization and Cycloisomerization of π-tethered ynamides: An expedient synthetic method to construct carbo-and heterocycles Synlett 28 2539; (h) Evano G, Michelet B and Zhang C 2017 The anionic chemistry of ynamides: A review C. R. Chimie 20 648; (i) Zhou B, Tan T-D, Zhu X-Q, Shang M and Ye L-W 2019 Reversal of Regioselectivity in Ynamide Chemistry ACS Catal. 9 6393
Lecomte M and Evano G 2016 Harnessing the Electrophilicity of Keteniminium Ions: A Simple and Straightforward Entry to Tetrahydropyridines and Piperidines from Ynamides Angew. Chem., Int. Ed. 55 4547
Selected examples: (a) Rettenmeier E, Schuster A M, Rudolph M, Rominger F, Gade C A and Hashmi A S K 2013 Gold Catalysis: Highly Functionalized Cyclopentadienes Prepared by Intermolecular Cyclization of Ynamides and Propargylic Carboxylates Angew. Chem., Int. Ed. 52 5880; (b) Kiruthika S E, Nandakumar A and Perumal P T 2014 Synthesis of Pyrrolo-/Indolo[1,2-a]quinolines and Naphtho[2,1-b]thiophenes from gem-Dibromovinyls and Sulphonamides Org. Lett. 16 4424 (c) Chen L, Cao J, Xu Z, Zheng Z-J, Cui Y-M, Xu L-W 2016 Lewis acid catalyzed [2+2] cycloaddition of ynamides and propargyl silyl ethers: synthesis of alkylidenecyclobutenones and their reactivity in ring-opening and ring expansion Chem. Commun. 52 9574
Selected recent examples: (a) Fugino D, Yorimitsu H and Osuka A 2014 Regiocontrolled Palladium-Catalyzed Arylative Cyclizations of Alkynols J. Am. Chem. Soc. 136 6255; (b) Karad S N and Liu R-S 2014 Regiocontrolled Gold-Catalyzed [2+2+2] Cycloadditions of Ynamides with Two Discrete Nitriles to Construct 4-Aminopyrimidine Cores Angew. Chem., Int. Ed. 53 9072; (c) Brioche J, Meyer C and Cossy J 2015 Org. Lett. 17 2800; (d) Adcock H V, Chatzopoulou E and Davies P W 2105 Divergent C¢H Insertion–Cyclization Cascades of N-Allyl Ynamides Angew. Chem., Int. Ed. 54 15525; (e) Straker R N, Peng Q, Mekareeya A, Paton R S and Anderson E A 2016 Computational ligand design in enantio- and diastereoselective ynamide [5+2] cycloisomerization Nat. Commun. 7 10109; (f) Cao Z, Zhu J, Liu L, Pang Y, Tian L, Sun X and Meng X 2019 AgNTf2-catalyzed formal [3 + 2] cycloaddition of ynamides with unprotected isoxazol-5-amines: efficient access to functionalized 5-amino-1H-pyrrole-3-carboxamide derivatives Beilstein J. Org. Chem. 15 2623; (g) Dutta S, Prabagar B, Vanjari R, Gandon V and Sahoo A K 2020 An Unconventional Sulfur-to-Selenium-to-Carbon Radical Transfer: Chemo-and Regioselective Cyclization of Yne-Ynamides Green Chem. 22 1113; (h) Yang M, Wang X and Zhao J 2020 Ynamide-Mediated Macrolactonization ACS Catal. 10 5230
Some relevant examples: (a) Smith D L, Goundry W R F and Lam H W 2012 Palladium-catalyzed hydroacyloxylation of ynamides Chem. Commun. 48 1505; (b) Yang Y, Wang L, Zhang J, Jin Y and Zhu G 2014 An unprecedented Pd-catalyzed trans-addition of boronic acids to ynamides Chem. Commun. 50 2347; (c) Yang Y, Wang L, Zhang F and Zhu G 2014 Preparation of (Z)-α,β-Disubstituted Enamides via Palladium-Catalyzed Addition of Boronic Acids to Ynamides J. Org. Chem. 79 9319; (d) Hu L, Xu S, Zhao Z, Yang Y, Peng Z, Yang M, Wang C and Zhao J 2016 Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis J. Am. Chem. Soc. 138 13135; (e) Tona V, Ruider S A, Berger M, Shaaban S, Padmanaban M, Xie L-G, González L and Maulide N Divergent ynamide reactivity in the presence of azides – an experimental and computational study 2016 Chem. Sci. 7 6032. (f) Li X Q, Jiang M Y, Zhan T Y, Cao W D and Feng X M 2020 Catalytic Asymmetric Three-component Hydroacyloxylation/1,4-Conjugate Addition of Ynamides Chem. Asian J. 15 1953
Recent examples: (a) Che J, Li Y, Zhang F, Zheng R, Bai Y and Zhu G 2014 Silver-promoted trans-hydrofluorination of ynamides: a regio- and stereoselective approach to (Z)-a-fluoroenamides Tetrahedron Lett. 55 6240; (b) Xu S, Liu J, Hu D, Bi X Metal-free hydroacyloxylation and hydration reactions of ynamides: synthesis of α-acyloxyenamides and N-acylsulfonamides 2015 Green Chem. 17 184; (c) Pirovano V, Negrato M, Abbiati G, Dell’Acqua M and Rossi E 2016 Gold-Catalyzed cis-Hydroarylation of Ynamides with Indoles: Regio and Stereoselective Synthesis of a Class of 2-Vinylindoles Org. Lett. 18 4798; (d) Shu C, Shen C-H, Wang Y-H, Li L, Li T, Lu X and Ye L-W 2016 Synthesis of 2-Aza-1,3-butadienes through Gold-Catalyzed Intermolecular Ynamide Amination/C−H Functionalization Org. Lett. 18 4630; (e) Sallio R, Corpet M, Habert L, Durandetti M, Gosmini C and Gillaizeau I 2017 Cobalt-Catalyzed Carbozincation of Ynamides J. Org. Chem. 82 1254
(a) He G, Qiu S, Huang H, Zhu G, Zhang D, Zhang R and Zhu H 2016 Cu(I)- or Ag(I)-Catalyzed Regio- and Stereocontrolled trans-Hydrofluorination of Ynamides Org. Lett. 18 1856; (b) Métayer B, Compain G, Jouvin K, Martin-Mingot A, Bachmann C, Marrot J, Evano G and Thibaudeau S 2015 Chemo- and Stereoselective Synthesis of Fluorinated Enamides from Ynamides in HF/Pyridine: Second-Generation Approach to Potent Ureas Bioisosteres J. Org. Chem. 80 3397; (c) Sato A H, Ohasi K and Iwasawa T 2013 Regio- and stereospecific synthesis of (E)-a-iodoenamide moieties from ynamides through iodotrimethylsilane-mediated hydroiodation Tetrahedron Lett. 54 1309; (d) Zhu G, Qiu S, Xi Y, Ding Y, Zhang D, Zhang R, He G and Zhu H 2016 (IPr)CuF-catalyzed α-site regiocontrolled trans-hydrofluorination of ynamides Org. Biomol. Chem. 14 7746; (e) Zeng X, Lu Z, Liu S, Hammond G B and Xu B 2017 Metal-free, Regio-, and Stereo-Controlled Hydrochlorination and Hydrobromination of Ynones and Ynamides J. Org. Chem. 82 13179; (f) Ohashi K, Mihara S, Sato A H, Ide M and Iwasawa T 2014 Synthesis of 1-haloethenamides from ynamide through halotrimethylsilane-mediated hydrohalogenation Tetrahedron Lett. 55 632; (g) Ide M, Ohashi K, Mihara S and Iwasawa T 2014 Regio- and stereoselective hydrohalogenation of ynamide components in 1,3-butadiynes with in situ generated HX Tetrahedron Lett. 55 2130; (h) Zeng X J, Li J L, Ng C K, Hammond G B and Xu B 2018 (Radio)Fluoro-Click Reaction Enabled by a Hydrogen Bonding Cluster Angew. Chem., Int. Ed. 57 2924 (i) Li J-L, Lin E, Han X-L, Li Q and Wang H 2019 Synthesis of α-Fluorinated Imides via Direct Fluorohydroxylation of Ynamides Org. Lett. 21 4255 (j) Kim S W, Uma T-W and Shin S 2017 Brønsted Acid-Catalyzed α-Halogenation of Ynamides from Halogenated Solvents and Pyridine-N-Oxides Chem. Commun. 53 1
(a) Hirano S, Tanaka R, Urabe H and Sato F 2004 Practical Preparation of N-(1-Alkynyl)sulfonamides and Their Remote Diastereoselective Addition to Aldehydes via Titanation Org. Lett. 6 727; (b) Saito N, Katayama T and Sato Y 2008 Nickel-Catalyzed Highly Regioselective Multicomponent Coupling of Ynamides, Aldehydes, and Silane: A New Access to Functionalized Enamides Org. Lett. 10 3829; (c) Graux L V, Clavier H and Buono G 2014 Palladium-Catalyzed Addition of 1,3-Diones to Ynamides: An Entry to Alkoxy-Substituted Enamides ChemCatChem. 6 2544
(a) Fadel A, Legrand F, Evano G and Rabasso N 2011 Highly Regio- and Stereoselective Nickel-Catalyzed Addition of Dialkyl Phosphites to Ynamides: an Efficient Synthesis of β-Aminovinylphosphonates Adv. Synth. Catal. 353 263; (b) Dwivedi V, Hari Babu M, Kant R and Sridhar Reddy M 2015 N-Substitution dependent stereoselectivity switch in palladium catalyzed hydroalkynylation of ynamides: a regio and stereoselective synthesis of ynenamides Chem. Commun. 51 14996; (c) Maity P, Klos M R and Kazmaier U 2013 Syntheses of α-Stannylated and α-Iodinated Enamides via Molybdenum-Catalyzed Hydrostannation Org. Lett. 15 6246
(a) Mulder J A, Kurtz K C M, Hsung R P, Coverdale H, Frederick M O, Shen L and Zificsak C A 2003 Highly Stereoselective Synthesis of Novel α-Haloenamides via a Mild and Efficient Hydrohalogenation of Ynamides Org. Lett. 5 1547; (b) Yabuuchi Y, Kuzuguchi T, Yoshimura T and Matsuo J 2016 Synthesis of α-Halo-γ-hydroxyenamides by Titanium Tetrahalide Mediated Addition of Aldehydes or Ketones to Ynamides Org. Lett. 18 4951; (c) Prabagar B, Nayak S, Mallick R K, Prasad R and Sahoo A K 2016 Triphenylphosphine promoted regio and stereoselective α-halogenation of ynamides Org. Chem. Front. 3 110; (d) Lu Z, Kong W, Yuan Z, Zhao X and Zhu G 2011 Synthesis of Multisubstituted Enamides via Pd-Catalyzed Chloroallylation of Ynamides J. Org. Chem. 76 8524; (e) Cao W, Chen P, Wang L, Wen H, Liu Y, Wang W and Tang Y 2018 A Highly Regio- and Stereoselective Syntheses of α-Halo Enamides, Vinyl Thioethers, and Vinyl Ethers with Aqueous Hydrogen Halide in Two-Phase Systems Org. Lett. 20 4507
(a) Nakanishi M, Minard C, Retailleau P, Cariou K and Dodd R H 2011 Copper(I) Catalyzed Regioselective Asymmetric Alkoxyamination of Aryl Enamide Derivatives Org. Lett. 13 5792; (b) Carbery D R 2008 Enamides: valuable organic substrates Org. Biomol. Chem. 3455; (c) Matsubara R and Kobayashi S 2008 Enamides and Enecarbamates as Nucleophiles in Stereoselective C–C and C–N Bond-Forming Reactions Acc. Chem. Res. 41 292; (d) Ma Z-X, Feltenberger J B and Hsung R P 2012 Total Syntheses of Chelidonine and Norchelidonine via an Enamide_Benzyne_[2 + 2] Cycloaddition Cascade Org. Lett. 14 2742; (e) Kuranaga T, Sesoko Y and Inoue M 2014 Cu-mediated enamide formation in the total synthesis of complex peptide natural products Nat. Prod. Rep. 31 514
(a) Gourdet B and Lam H W 2010 Catalytic Asymmetric Dihydroxylation of Enamides and Application to the Total Synthesis of (+)-Tanikolide Angew. Chem., Int. Ed. 49 8733; (b) St. Denis J D, Zajdlik A, Tan J, Trinchera P, Lee C F, He Z, Adachi S and Yudin A K 2014 Boron-Containing Enamine and Enamide Linchpins in the Synthesis of Nitrogen Heterocycles J. Am. Chem. Soc. 136 17669; (c) Song Z, Lu T, Hsung R P, Al-Rashid Z F, Ko C and Tang Y 2007 Stereoselective Simmons–Smith Cyclopropanation of Chiral Enamides Angew. Chem., Int. Ed. 46 4069
(a) Henderson W E and Gangloff W 1916 The action of anhydrous aluminium chloride upon unsaturated compounds J. Am. Chem. Soc. 18 1382; (b) Wang C and Xi Z 2007 Co-operative effect of Lewis acids with transition metals for organic synthesis Chem. Soc. Rev. 36 1395
(a) Yang Y, Liu H, Peng C, Wu J, Zhang J, Qiao Y, Wang X-N and Chang J 2016 AlCl3-Catalyzed Annulations of Ynamides Involving a Torquoselective Process for the Simultaneous Control of Central and Axial Chirality Org. Lett. 18 5022; (b) Oppenheimer J, Johnson W L, Tracey M R, Hsung R P, Yao P-Y, Liu R and Zhao K 2007 A Rhodium(I)-Catalyzed Demethylation-Cyclization of o-Anisole-Substituted Ynamides in the Synthesis of Chiral 2-Amido Benzofurans Org. Lett. 9 2361; (c) Chen L, Yu L, Deng Y, Cui Y, Bian G and Cao J 2016 Synthesis of α-haloenamides via zinc halide mediated direct addition of benzhydryl halides to ynamides Org. Biomol. Chem. 14 564
Our earlier work on ynamides: (a) Siva Reddy A, Nagarjuna Reddy M and Kumara Swamy K C 2014 A simple copper-catalysed tandem cyclisation of ynamides leading to triazolo-1,2,4-benzothiadiazine-1,1-dioxides in PEG-400 medium RSC Adv. 4 28359; (b) Siva Reddy A and Kumara Swamy K C 2015 Use of Elemental Sulfur or Selenium in a Novel One-Pot Copper-Catalyzed Tandem Cyclization of Functionalized Ynamides Leading to Benzosultams Org. Lett. 17 2996; (c) Siva Reddy A, Kumari, A L S, Saha S and Kumara Swamy K C 2016 Palladium-Catalyzed Tandem-Cyclization of Functionalized Ynamides: An Approach to Benzosultams Adv. Synth. Catal. 358 1625; (d) Kumari A L S, Siva Reddy A and Kumara Swamy K C 2016 Transition Metal-Free Cascade Cyclization of Epoxy-Ynamides: To Go for 1,3-Oxazines or 1,4-Oxazines? Org. Lett. 18 5752; (e) Anitha M, Shankar M and Kumara Swamy K C 2019 Reactivity of epoxy-ynamides with metal halides: nucleophile (Br/Cl/OH)-assisted tandem intramolecular 5-exo-dig or 6-endo-dig cyclisation and AgF2-promoted oxidation Org. Chem. Front. 6 1133; (f) Siva Reddy A and Kumara Swamy K C 2017 Ethanol as a Hydrogenating Agent: Palladium-Catalyzed Stereoselective Hydrogenation of Ynamides To Give Enamides Angew. Chem. Int. Ed. 56 6984
(a) Ho M L, Flynn A B and Ogilvie W W 2007 Single-Isomer Iodochlorination of Alkynes and Chlorination of Alkenes Using Tetrabutylammonium Iodide and Dichloroethane J. Org. Chem. 72 977; (b) Ide M, Yauchi Y and Iwasawa T 2014 Regio-, and Stereoselective Iodobromination of Ynamides for Synthesis of (E)-1-Bromo-2-iodoenamides Eur. J. Org. Chem. 3262
(a) Zhang Y, Hsung R P, Tracey M R, Kurtz K C M and Vera E L 2004 Copper Sulfate-Pentahydrate-1,10-Phenanthroline Catalyzed Amidations of Alkynyl Bromides. Synthesis of Heteroaromatic Amine Substituted Ynamides Org. Lett. 6 1151; (b) Dateer R B, Shaibu B S and Liu R S 2012 Gold-Catalyzed Intermolecular [4+2] and [2+2+2] Cycloadditions of Ynamides with Alkenes Angew. Chem., Int. Ed. 51 113
For another recent example, see: Anitha M and Kumara Swamy K C 2019 Highly functionalised (γ-azido/γ-fluoro-β-iodo/) vinyl derivatives from phosphorus based allenes or allenoates: I···O halogen bonding interactions Org. Biomol. Chem. 17 5736
Acknowledgements
We thank the Department of Science and Technology (DST, New Delhi) and University Grants Commission (UGC, New Delhi) for support. ASR, MA and Suraj thank UGC (New Delhi) for fellowships. KCK thanks SERB for the J. C. Bose fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siva Reddy, A., Anitha, M., Suraj et al. Reactivity of ynamides with AlCl3 and ICl: Ready access to (E)-α-chloroenamides and (E/Z)-α-chloro-β-iodo-enamides. J Chem Sci 133, 6 (2021). https://doi.org/10.1007/s12039-020-01880-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01880-4