Abstract
Density function theory (DFT) was performed to study the structures and binding energies of the 2-aminopyridine(2-AP)/2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) complex. Four distinct stable conformers (denoted as S1–S4) were located and the bonding characteristics of these conformers were investigated with NBO and AIM analysis. It was revealed that S1 is formed by the edge(2-AP)-to-face(DDQ) linkage, which is stabilized by a moderate \({\upsigma }\)–\({\uppi }\) interaction and hydrogen binding interaction. S2 and S3 are formed by the face(2-AP)-to-face(DDQ) linkage through \({\upsigma }\)–\({\uppi }\) and \({\uppi }\)–\({\uppi }\) interactions, and S4 is constructed by the edge(2-AP)-to-edge(DDQ) linkage through halogen bonding and hydrogen bonding interactions. The electronic excitation energies of the two stable conformers, S2 and S3, were calculated with time-dependent DFT (TDDFT), which revealed that the 2-AP\(({\uppi })\) \(\rightarrow \) DDQ(\({\uppi }\)*) charge transfer transitions in conformers S2 and S3 contribute to the two new charge transfer absorption bands which were experimentally observed at 598 nm and 557 nm for the 2-AP/DDQ complex in chloroform.
Graphical Abstract
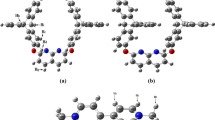
Four probable structures of the 2-AP/DDQ complex were located by DFT calculation. The bonding characteristics between the two molecules of the complex were investigated by NBO and AIM analysis. Two new absorption bands observed in the UV–Vis absorption spectrum of the complex were attributed according to the TDDFT results.





Similar content being viewed by others
References
Horiuchi S, Hasegawa T and Tokura Y 2006 Molecular donor–acceptor compounds as prospective organic electronics materials J. Phys. Soc. Jpn. 75 051016
Goetz K P, Vermeulen D, Payne M E, Kloc C, McNeil L E and Jurchescu O D 2014 Charge-transfer complexes: New perspectives on an old class of compounds J. Mater. Chem. C 2 3065
Das A and Ghosh S 2014 Supramolecular assemblies by charge–transfer interactions between donor and acceptor chromophores Angew. Chem. Int. Ed. 53 2038
Budzáka Š, Mach P, Juhász G, Medved’ M and Kysel’ O 2015 Theoretical study of charge transfer complexes between antithyroid thioamides and TCNE: Thermodynamics of the complex formation Comput. Theor. Chem. 1051 129
Habeeb M M, Al-Attas A S and Al-Raimi D S 2015 Spectroscopic studies and molecular orbital calculations of charge transfer complexation between 3,5-dimethylpyrazole with DDQ in acetonitrile Spectrochim. Acta A 142 196
Li Z-Y, Wang H-L, He T-J, Liu F-C and Chen D-M 2006 Theoretical study on the structure and UV–visible spectrum of pyridine–chloranil charge-transfer complex J. Mol. Struct.-THEOCHEM 778 69
Al-Ahmary K M, El-Kholy M M, Al-Solmy I A and Habeeb M M 2013 Spectroscopic studies and molecular orbital calculations on the charge transfer reaction between DDQ and 2-aminopyridine Spectrochim. Acta A 110 343
Bazzi H S, Mostafa A, AlQaradawi S Y and Nour E M 2007 Synthesis and spectroscopic structural investigations of the charge-transfer complexes formed in the reaction of 2,6-diaminopyridine with \({\uppi }\)-acceptors TCNE, chloranil, and DDQ J. Mol. Struct. 842 1
Mostafa A and Bazzi H S 2009 Synthesis and spectroscopic studies of the charge transfer complexes of 2- and 3-aminopyridine Spectrochim. Acta A 74 180
Sobolewskia A L and Domcke W 2003 Ab initio study of the excited-state coupled electron–proton-transfer process in the 2-aminopyridine dimmer Chem. Phys. 294 73
Müller A, Talbot F and Leutwyler S 2002 Hydrogen bond vibrations of 2-aminopyridine\(\cdot \) 2-pyridone, a Watson-Crick analogue of adenine \(\cdot \) uracil J. Am. Chem. Soc. 24 14486
Rabenstein B, Ullmann G M and Knapp E W 2000 Electron transfer between the quinones in the photosynthetic reaction center and its coupling to conformational changes Biochemistry 39 10487
Lieffrig J, Jeannin O, Shin K S, Auban-Senzier P and Fourmigué M 2002 Halogen bonding interactions in DDQ charge transfer salts with iodinated TTFs Crystals 2 327
Zhang Y-H, Zhou K-G, Xie K-F, Zeng J, Zhang H-L and Peng Y 2010 Tuning the electronic structure and transport properties of graphene by noncovalent functionalization: Effects of organic donor, acceptor and metal atoms Nanotechnology 21 065201
Hohenstein E G, Chill S T and Sherrill S D 2008 Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules J. Chem. Theory Comput. 4 1996
Rosokha S V and Loboda E A 2015 Interplay of halogen and \({\uppi }{-}{\uppi }\) charge-transfer bondings in intermolecular associates of bromo- or iododinitrobenzene with tetramethyl-p-phenylenediamine J. Phys. Chem. A 119 3833
Karir G, Fatima M and Viswanathan K S 2016 The elusive \(\equiv \text{ C }{-}\text{ H }{\cdots } \text{ O }\) complex in the hydrogen bonded systems of phenylacetylene: A matrix isolation infrared and Ab Initio study J. Chem. Sci. 128 1557
Budzák Š, Mach P, Medved’a M and Kysel’a O 2015 Critical analysis of spectral solvent shifts calculated by the contemporary PCM approaches of a representative series of charge-transfer complexes between tetracyanoethylene and methylated benzenes Phys. Chem. Chem. Phys. 17 17618
Boys S F and Bernardi F 1970 The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 19 553
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint Chem. Rev. 88 899
Biegler–KÄonig F, SchÄonbohm J and Bayles D 2002 Update of the AIM 2000-Program for atoms in molecules J. Comput. Chem. 23 1489
Tomasi J, Mennucci B and Cances E 1999 The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level J. Mol. Struct.-THEOCHEM 464 211
Tomasi J, Mennucci B and Cammi R 2005 Quantum Mechanical Continuum Solvation Models Chem. Rev. 105 2999
Frisch M J et al. 2009 Gaussian 09, Revision D.01; Gaussian, Inc., Wallingford, CT
Bondi A 1964 Van der waals volumes and radii J. Phys. Chem. 68 441
Misiaszeka T and Czyżnikowska Z 2014 The nature of interactions in nicotinamide crystal J. Mol. Graph. Model. 51 73
Mohan N and Suresh C H 2014 A molecular electrostatic potential analysis of hydrogen, halogen, and dihydrogen bonds J. Phys. Chem. A 118 1697
Szatylowicz H, Jezierska A and Sadlej-Sosnowska N 2016 Correlations of NBO energies of individual hydrogen bonds in nucleic acid base pairs with some QTAIM parameters Struct. Chem. 27 367
Li H-Y, Lu Y-X, Liu Y-T, Zhu X, Liu H-L and Zhu W-L 2012 Interplay between halogen bonds and \({\uppi }\)–\({\uppi }\) stacking interactions: CSD search and theoretical study Phys. Chem. Chem. Phys. 14 9948
Shyam Vinod Kumar P, Raghavendra V and Subramanian V 2016 Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding J. Chem. Sci. 128 1527
Acknowledgements
This project was financially supported by the Department of Education of Zhejiang Province (Y201330088).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H. Theoretical study on the molecular structure, intermolecular interaction and spectral features of 2-aminopyridine/ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone complex. J Chem Sci 129, 775–782 (2017). https://doi.org/10.1007/s12039-017-1277-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1277-3