Abstract
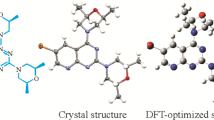
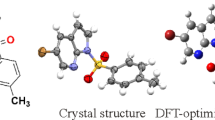
Novel pyrrolo[1′,5′-a]-1,8-naphthyridine compounds (L1-L4) have been synthesized through a facile one-pot process by the reaction of the corresponding 1,8-naphthyridines with aliphatic anhydride. The structures were established by spectroscopic data. Further, X-ray crystal analysis of 7-diacetamino-2,4-dimethy-1,8-naphthyridine (L1) identifies its molecular structure and reveals π- π stacking. The synthetic mechanisms for L2, L3 were studied by density functional theory calculations. And a comprehensive study of spectroscopic properties involving experimental data and theoretical studies is presented. L1 exhibited electronic absorption spectrum with λ max at ∼320 nm. L2-L4 exhibited similar electronic absorption spectra with λ max at ∼390 nm that is tentatively assigned to π→π* transition. The assignment was further supported by density functional theory (DFT) calculations.

Novel pyrrolo[1′,5′-a]-1,8-naphthyridine compounds have been synthesized through a facile one-pot process. Further, X-ray crystal analysis and spectroscopic properties are presented.









Similar content being viewed by others
References
Zou Y Q, Lu L Q, Fu L, Chang N J, Rong J, Chen J R and Xiao W J 2011 Angew. Chem. Int. Ed. 50 7171
Feng C, Yan Y, Zhang Z, Xu K and Wang Z 2014 Org. Biomol. Chem. 12 4837
Xiang L, Yang Y, Zhou X, Liu X, Li X, Kang X and Huang G 2014 J. Org. Chem. 79 10641
Reissert A 1893 Ber. Dtsch. Chem. Ges. 26 2137
Bera J K, Sadhukhan N and Majumdar M 2009 Eur. J. Inorg. Chem. 4023
Mahalingam M, Mohan P S, Gayathri K, Gomathi R and Subhapriya P 2013 J. Chem. Sci. 125 1015
Kota T P and Kollipara M R 2014 J. Chem. Sci. 126 1143
Wang C X, Sato Y, Kudo M, Nishizawa S and Teramae N 2012 Chem-Eur. J. 18 9481
Hu S Z and Chen C F 2010 Chem. Commun. 46 4199
Gan Q, Ferrand Y, Chandramouli N, Kauffmann B, Aube C, Dubreuil D and Huc I 2012 J. Am. Chem. Soc. 134 15656
Yamazaki H, Hakamata T, Komi M and Yagi M 2011 J. Am. Chem. Soc. 133 8846
Tseng H W, Zong R, Muckerman J T and Thummel R 2008 Inorg. Chem. 47 11763
Bhuniya D, Umrani D, Dave B, Salunke D, Kukreja G, Gundu J, Naykodi M, Shaikh N S, Shitole P and Kurhade S 2011 Bioorg. Med. Chem. Lett. 21 3596
Du M L, Hu C Y, Wang L F, Li C, Han Y Y, Gan X and Fu W F 2014 J. Chem. Soc., Dalton Trans. 43 13924
Fernández-Mato A, Quintela J M and Peinador C 2012 New J. Chem. 36 1634
Wu Y Y, Chen Y, Gou G Z, Mu W H, Lv X J, Du M L and Fu W F 2012 Org. Lett. 14 5226
Boekelheide V and Windgassen R J 1959 J. Am. Chem. Soc. 81 1456
Jones G and Stanyer J 1969 J. Chem. Soc. C 6 901
Zhao X J, Chen Y, Fu W F and Zhang J B 2007 Synth. Commun. 37 2145
Crosby G A and Demas J N 1971 J. Phys. Chem. 75 991
Sheldrich G M 1997 SHELX-97, Program for the refinement of crystal structures, University of Gottingen, Germany
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J and Fox D J 2010 Gaussian 09, Revision B.01, (Gaussian, Inc.: Wallingford CT)
Raissi H, Yoosefian M, Moshfeghi E and Farzad F 2012 J. Chem. Sci. 124 731
Tiwary A S and Mukherjee A K 2013 J. Chem. Sci. 125 905
Becke A D 1988 Phys. Rev. A 38 3098
Becke A D 1993 J. Chem. Phys. 98 5648
Ghosh S, Girish K V S and Ghosh S 2013 J. Chem. Sci. 125 933
Gou G Z, Zhou B, Shi L, Chi S M, Mang C Y and Liu W 2016 Theor. Chem. Acc. 135 1
Hehre W J, Ditchfield R and Pople J A 1972 J. Chem. Phys. 56 2257
Francl M M, Pietro W J, Hehre W J, Binkley J S, Gordon M S, DeFrees D J and Pople J A 1982 J. Chem. Phys. 77 3654
Krishnan R, Binkley J S, Seeger R and Pople J A 1980 J. Chem. Phys. 72 650
Gou G Z, Bo Z, Shi L, Xu S J, Yan H P, Liu W and Mang C Y 2015 Indian J. Chem. A 54 1017
Miertuš S, Scrocco E and Tomasi J 1981 Chem. Phys. 55 117
Gou G Z, Zhou B, Shi L, Chi S M, Chen X L and Liu W 2015 Chin. J. Chem. Phys. 28 695
Henry R and Hammond P 1977 J. Heterocyclic Chem. 14 1109
Brown E V 1965 J. Org. Chem. 30 1607
Abranyi-Balogh P, Mucsi Z, Csizmadia I G, Dancso A, Keglevich G and Milen M 2014 Tetrahedron 70 9682
Acknowledgements
This work is supported by the National Natural Science Foundation of China (61361002, 21262049 and 21461007), the “Chun Hui” Plan of Chinese Ministry Education (Z2011125), the Scientific Research Foundation of Education Department of Yunnan Province (2013FZ121), the General Program of Yunnan Provincial Education Departmen (2015Y455), the Chemistry of Key Construction Disciplines for Master Degree Program in Yunnan (HXZ1303) and the Educational Reform Program of Honghe University (JJJG1412).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information (SI)
Supplementary information associated with this article, i.e., experimental procedures, characterization data, computational details, additional spectral data, and crystallographic data (CIF) are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
GOU, GZ., ZHOU, B., YAN, HP. et al. Synthesis, Spectroscopic Properties and DFT Calculation of Novel Pyrrolo[1′,5′-a]-1,8-naphthyridine Derivatives through a Facile One-pot Process. J Chem Sci 128, 1813–1821 (2016). https://doi.org/10.1007/s12039-016-1163-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1163-4