Abstract
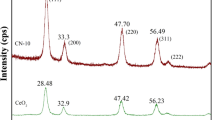
The direct synthesis of dimethyl carbonate (DMC) from carbon dioxide (CO2) and methanol is an attractive approach towards conversion of the greenhouse gas - CO2 into value-added chemicals and fuels. Ceria (CeO2) catalyzes this reaction. But the conversion efficiency of CeO2 is enhanced when the byproduct water in the reaction medium is separated by employing trapping agents like 2-cyanopyridine (2-CP). In this work, the influence of morphology of CeO2 on the direct synthesis of DMC in presence of 2-CP is reported. CeO2 catalysts of cube, rod, spindle and irregular morphology (Ce - C, Ce - R, Ce - S and Ce - N, respectively) were prepared, characterized and studied as catalysts in the said reaction conducted in a batch mode. Among all, Ce - S shows superior catalytic performance with nearly 100 mol% of DMC selectivity. Catalytic activity correlates with the concentration of acid and base sites of medium strength as well as defect sites. Ce - S has an optimum number of these active sites and thereby shows superior catalytic performance.

Direct synthesis of dimethyl carbonate from CO2 and methanol in presence of 2-cyanopyridine as water trapping agent was studied with CeO2 of different morphologies as a catalyst. Catalytic activity correlates with the amount of acid/base sites of medium strength, defect sites and exposed (111) facets. CeO2 with spindle morphology shows superior catalytic performance compared to rod, cube and irregular or ‘no-definite’ shape morphologies.








Similar content being viewed by others
References
http://www.cop21.gouv.fr/en/why-2c/ (accessed on 01 January 2016)
Arresta M 2010 In Carbon dioxide as a chemical feedstock (Weinheim: Wiley-VcH Verlag)
Unnikrishnan P and Srinivas D 2015 In Industrial catalysis and separations: Innovations for process intensification (NJ, USA: Apple Academic)
Sakakura T, Choi J C and Yasuda H 2007 Chem. Rev. 107 2365
Honda M, Tamura M, Nakagawa Y and Tomishige K 2014 Catal. Sci. Technol. 4 2830
Tundo P and Selva M 2002 Acc. Chem. Res. 35 706
Fukuoka S, Fukawa I, Tojo M, Oonishi K, Hachiya H, Aminaka M, Hasegawa K and Komiya K 2010 Catal. Surv. Asia 14 146
Unnikrishnan P and Srinivas D 2015 J. Mol. Catal. A: Chem. 398 42
Huang S, Yan B, Wang S and Ma X 2015 Chem. Soc. Rev. 44 3079
Eta V, Mäki-Arvela P, Wärnå J, Salmi T, Mikkola J P and Murzin D Y 2011 Appl. Catal. A: Gen. 404 39
Tomishige K and Kunimori K 2002 Appl. Catal. A: Gen. 237 103
Jung K T and Bell A T 2001 J. Catal. 204 339
Ikeda Y, Asadullha M, Fujimoto K and Tomishige K 2001 J. Phys. Chem. B 105 10653
Wang S, Zhao L, Wang W, Zhao Y, Zhang G, Ma X and Gong J 2013 Nanoscale 5 5582
Unnikrishnan P, Varhadi P and Srinivas D 2013 RSC Adv. 3 23993
Vinodkumar T, Naga Durgasri D, Swamy M and Reddy B M 2015 J. Chem. Sci. 127 1145
Naga Durgasri D, Vinodkumar T and Reddy B M 2014 J. Chem. Sci. 126 429
Dutta G, Gupta A, Waghmare U V and Hegde M S 2011 J. Chem. Sci. 123 509
Sanjaykumar S R, Mukri B D, Patil S, Madras G and Hegde M S 2011 J. Chem. Sci. 123 47
Gayen A, Baidya T, Ramesh G S, Srihari R and Hegde M S 2006 J. Chem. Sci. 118 47
Mishra B G, Ranga Rao G and Poongodi B 2003 Proc. Ind. Acad. Sci. (J. Chem. Sci.) 115 561
Honda M, Kuno S, Sonehara S, Fujimoto K -i, Suzuki K, Nakagawa Y and Tomishige K 2011 Chem. Cat. Chem. 3 365
Honda M, Tamura M, Nakagawa Y, Sonehara S, Suzuki K, Fujimoto K and Tomishige K 2013 Chem. Sus. Chem. 6 1341
Wang S P, Zhou J J, Zhao S Y, Zhao Y J and Ma X B 2015 Chin. Chem. Lett. 26 1096
http:// www.asahi-kasei.co.jp/asahi/en/news/2014/e150119.html (accessed on 16 April 2015)
Fan T, Zhang L X, Jiu H F, Sun Y X, Liu G D, Sun Y Y and Su Q L 2010 Micro Nano Lett. 5 230
Nakajima A, Yoshihara A and Ishigame M 1994 Phys. Rev. B 50 297
Wu Z, Li M, Howe J, Meyer H M and Overbury S H 2010 Langmuir 26 16595
Eta V, Maki-Arvela P, Leino A R, Kordas K, Salmi T, Murzin D Y and Mikkola J P 2010 Ind. Eng. Chem. Res. 49 9609
Acknowledgements
P.U. acknowledges CSIR, New Delhi for the fellowship. This work forms a part of the Project “TapCoal (CSC 0102)” sponsored by CSIR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
HRTEM images of Ce - N sample and catalytic activity data of Ce - S as a function of reaction temperature and reaction time are available as supporting information on the website of Journal of Chemical Sciences at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
P, U., DARBHA, S. Direct synthesis of dimethyl carbonate from CO2 and methanol over CeO2 catalysts of different morphologies. J Chem Sci 128, 957–965 (2016). https://doi.org/10.1007/s12039-016-1094-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1094-0