Abstract
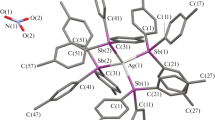
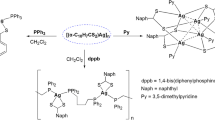
Three dinuclear, ionic and homoleptic metallacycles decorated with free thiophenyl or thiomethyl units were synthesized using AgOS(O 2)CF 3 and benzimidazole derived ditopic N-donor ligands at ambient condition. The complexes were characterized by analytical and spectroscopic (IR and 1H NMR) techniques and two of the complexes were structurally characterised through single crystal X-ray diffraction.

A new type of silver metallacycles with covalently attached free thiophenyl/thiomethyl units was synthesized using AgOS(O)2CF3 and ditopic ligands and characterized by using crystallographic and spectroscopic techniques.






Similar content being viewed by others
References
(a) Khlobystov A N, Blake A J, Champness N R, Lemenovskii D A, Majouga A G, Zyk N V and Schröder M 2001 Coord. Chem. Rev. 222 155; (b) Steel P J and Fitchett C M 2008 Coord. Chem. Rev. 252 990; (c) Young A G and Hanton L R 2008 Coord. Chem. Rev. 252 1346; (d) Slenters T V, Sagué J L, Brunetto P S, Zuber S, Fleury A, Mirolo L, Robin A Y, Meuwly M, Gordon O, Landmann R, Daniels A U and Fromm K M 2010 Materials 3 3407; (e) Burgess J and Steel P J 2011 Coord. Chem. Rev. 255 2094; (f) Fromm K M 2013 Appl. Organomet. Chem. 27 683
(a) Grapperhaus C A, Li M and Mashuta M S 2002 Chem. Commun. 1792; (b) Hannon M J, Painting C L, Plummer E A, Childs L J and Alcock N W 2002 Chem. Eur. J. 8 2225; (c) Steel P J and Sumby C J 2002 Chem. Commun. 322; (d) Bu X-H, Xie Y-B, Li J-R and Zhang R-H 2003 Inorg. Chem. 42 7422; (e) Dong Y-B, Zhang H-Q, Ma J-P, Huang R-Q and Su C-Y 2005 Cryst. Growth Des. 5 1857; (f) Li X-P, Zhang J-Y, Pan M, Zheng S-R, Liu Y and Su C-Y 2007 Inorg. Chem. 46 4617
(a) Caradoc-Davies P L and Hanton L R 2003 Dalton Trans. 1754; (b) Sague J L, Meuwly M and Fromm K M 2008 Cryst. Eng. Commun. 10 1542; (c) Deng Z-P, Zhu L-N, Gao S, Huo L-H and Ng S W 2008 Cryst. Growth Des. 8 3277; (d) Wei W, Wu M, Huang Y, Gao Q, Zhang Q, Jiang F and Hong M 2009 Cryst. Eng. Commun. 11 576; (e) Kilpin K J, Gower M L, Telfer S G, Jameson G B and Crowley J D 2011 Inorg. Chem. 50 1123; (f) Kim C W, Noh T H and Jung O-S 2011 Inorg. Chim. Acta 365 496; (g) Lee H, Kim E J, Ahn J, Noh T H and Jung O-S 2012 J. Mol. Struc. 1010 111; (h) Wei W, Yu H, Jiang F, Liu B, Ma J and Hong M 2012 Cryst. Eng. Commun. 14 1693; (i) Wan C-Q, Al-Thabaiti S A, Chen X-D and Mak T C W 2013 Eur. J. Inorg. Chem. 5265; (j) Joardar S, Roy, Samanta S and Dutta A M 2015 J. Chem. Sci. 127 1819; (k) Li Y, Zhang W L, Du H J, Wang C H, Lu Y B and Niu Y Y 2015 J. Chem. Sci. 127 1513; (l) Gupta A, Srivastava A K and Boomishankar R 2015 J. Chem. Sci. 127 619
(a) Dias H V R, Diyabalanage H V K and Gamage C S P 2005 Chem. Commun. 1619; (b) Hettiarachchi C V, Rawashdeh-Omary M A, Korir D, Kohistani J, Yousufuddin M and Dias H V R 2013 Inorg. Chem. 52 13576
(a) Reger D L, Foley E A and Smith M D 2010 Inorg. Chem. 49 234; (b) Gardinier J R, Tatlock H M, Hewage J S and Lindeman S V 2013 Cryst. Growth Des. 13 3864
(a) Song R-F, Xie Y-B and Bu X-H 2003 J. Mol. Struct. 657 311; (b) Wu C-J, Lin C-Y, Cheng P-C, Yeh C-W, Chen J-D and Wang J-C 2011 Polyhedron 30 2260
du Plessis M, Smith V J and Barbour L J 2014 Cryst. Eng. Commun. 16 4126
(a) Su C-Y, Cai Y-P, Chen C-L, Smith M D, Kaim W and zur Loye H-C 2003 J. Am. Chem. Soc. 125 8595; (b) Chen C-L, Tan H-Y, Yao J-H, Wan Y-Q and Su C-Y 2005 Inorg. Chem. 44 8510; (c) Raehm L, Mimassi L, Guyard-Duhayon C, Amouri H and Rager M N 2003 Inorg. Chem. 42 5654
(a) Maekawa M, Kitagawa S, Kuroda-Sowa T and Munakata M 2006 Chem. Commun. 2161; (b) Dash C, Mobin S M and Ghosh P 2011 J. Chem. Sci. 123 97
Zhou J, Liu X, Zhang Y, Li B and Zhang Y 2006 Inorg. Chem. Commun. 9 216
Laye R H 2007 Inorg. Chim. Acta 360 439
(a) Chen C-L, Yu Z-Q, Zhang Q, Pan M, Zhang J-Y, Zhao C-Y and Su C-Y 2008 Cryst. Growth Des. 8 897; (b) Mudsainiyan R, Jassal A K, Arora M and Chawla S K 2015 J. Chem. Sci. 127 849; (c) Murugavel R, Anantharaman G, Krishnamurthy D, Sathiyendiran M and Walawalkar M G 2000 Proc. Indian Acad. Sci. (J. Chem. Sci.) 112 273
Purohit C S and Verma S 2007 J. Am. Chem. Soc. 129 3488
Pogozhev D, Baudron S A and Hosseini M W 2011 Dalton Trans. 40 437
(a) Shankar B, Rajakannu P, Kumar S, Gupta D, Kannan T and Sathiyendiran M 2011 Inorg. Chem. Commun. 14 374; (b) Gupta D, Rajakannu P, Shankar B, Shanmugam R, Hussain F, Sarkar B and Sathiyendiran M 2011 Dalton Trans. 40 5433; (c) Shankar B, Hussain F and Sathiyendiran M 2012 J. Organomet. Chem. 719 26; (d) Rajakannu P, Hussain F, Shankar B and Sathiyendiran M 2012 Inorg. Chem. Commun. 26 46; (e) Shankar B, Elumalai P, Jackmil P J, Kumar P, Singh S and Sathiyendiran M 2013 J. Organomet. Chem. 743 109; (f) Shankar B, Elumalai P, Hussain F and Sathiyendiran M 2013 J. Organomet. Chem. 732 130; (g) Rajakannu P, Elumalai P, Mobin S M, Lu K-L and Sathiyendiran M 2013 J. Organomet. Chem. 743 17; (h) Shankar B, Sahu S, Deibel, N, Schweinfurth D, Sarkar B, Elumalai P, Gupta D, Hussain F, Krishnamoorthy G and Sathiyendiran M 2014 Inorg. Chem. 53 922; (i) Gupta D, Rajakannu P, Shankar B, Hussain F and Sathiyendiran M 2014 J. Chem. Sci. 126 1501; (j) Rajakannu P, Mobin S M and Sathiyendiran M 2014 J. Organomet. Chem. 771 68; (k) Shankar B, Elumalai P, Sathiyashivan D and Sathiyendiran M 2014 Inorg. Chem. 53 10018; (l) Shankar B, Elumalai P, Shanmugam R, Singh V, Masram D T and Sathiyendiran M 2013 Inorg. Chem. 52 10217; (m) Elumali P, Kanagaraj R, Marimuthu R, Shankar B, Kalita A C and Sathiyendiran M 2015 Dalton Trans. 44 11274
(a) ENHANCE, Oxford Xcalibur Single Crystal Diffractometer, version 1.171.34.40, Oxford Diffraction Ltd, Oxford, U.K., 2006; (b) CrysAlisPro, version 1.171.34.40, Oxford Diffraction Ltd, Oxford, U.K., 2006; (c) SMART, Bruker Molecular Analysis Research Tool, version 5.0, Bruker Analytical X-ray Systems, Madison, WI 2000; (d) SAINT-NT, version 6.04, Bruker Analytical X-ray Systems, Madison, WI 2001; (e) Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla M C, Polidori G and Camalli M 1994 J. Appl. Crystallogr. 27 435; (f) Sheldrick G M SHELXL-97, Program for crystal structure refinement, University of Gottingen, Gottingen, Germany 1997; (g) Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Acknowledgements
We thank USIC & Department of Chemistry, University of Delhi, and School of Chemistry, University of Hyderabad for financial support and instrumentation facilities. SY thanks UGC for Junior Research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Copies of NMR, mass spectra and crystallographic data table are available at www.ias.ac.in/chemsci. CCDC 1400518 and 1400519, contain the supplementary crystallographic data for 1 and 3. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223-336 033; or e-mail: deposit@ccdc.cam.ac.uk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
YADAV, S., GUPTA, D. & SATHIYENDIRAN, M. Silver(I) based dinuclear metallacycles with free thiophenyl/thiomethyl units. J Chem Sci 128, 177–184 (2016). https://doi.org/10.1007/s12039-015-1027-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-1027-3