Abstract
Perioperative neurocognitive dysfunction is a significant concern for population health, impacting postoperative recovery and increasing the financial burden on patients. With an increasing number of surgical procedures being performed, the prevention and management of perioperative neurocognitive dysfunction have garnered significant attention. While factors such as age, lifestyle, genetics, and education are known to influence the development of cognitive dysfunction, recent research has highlighted the role of the gut microbiota in neurological health. An increased abundance of pro-inflammatory gut microbiota can trigger and worsen neuroinflammation, neuronal cell damage, and impaired cellular autophagy. Moreover, the inflammation-promoting gut microbiota can disrupt immune function, impair neuroautophagy, and affect the production and circulation of extracellular vesicles and neurotransmitters. These factors collectively play a role in the onset and advancement of cognitive impairment. This narrative review delves into the molecular mechanisms through which gut microbiota and their derivatives contribute to cognitive impairment, focusing on the impact of anesthesia surgery, changes in gut microbial populations, and perioperative cognitive impairment associations. The study suggests that alterations in the abundance of various bacterial species and their metabolites pre- and post-surgery may be linked to postoperative cognitive impairment. Furthermore, the potential of probiotics or prebiotics in addressing cognitive impairment is discussed, offering a promising avenue for investigating the treatment of perioperative neurocognitive disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
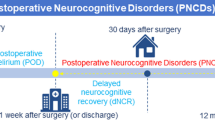
Perioperative neurocognitive dysfunction (PND), which encompasses preoperative cognitive dysfunction, postoperative delirium, delayed neurocognitive recovery, and postoperative neurocognitive disorder, commonly affects elderly patients [1, 2]. Age is a major risk factor for PND, with cognitive impairment occurring more frequently in elderly patients than in younger patients undergoing surgery [3]. Patients who undergo cardiovascular and orthopedic surgeries have a greater incidence of postoperative cognitive disorders, with approximately 40% of cardiovascular surgery patients experiencing PND [4], while approximately 23.8% of noncardiovascular surgery patients experience postoperative cognitive disorders [5]. Factors such as education level, type of anesthesia, and postoperative pain also contribute to the risk of PND [6,7,8,9,10]. The pathogenesis of PND remains unclear, with current theories focusing on factors such as intraoperative and postoperative microembolism, neuroinflammation, oxidative stress, neuronal demodulation, synaptic structural plasticity damage, abnormal Tau protein modification, and neurotrophic factor deprivation [11, 12]. Interestingly, the study by Glumac et al. [13] has shown that preoperative administration of dexamethasone before cardiac surgery significantly decreases the occurrence of postoperative neurocognitive deficits in patients. The suggested mechanism for this protective effect is by reducing neuroinflammatory responses and enhancing tolerance to perioperative stress. This evidence highlights the crucial role of neuroinflammation in the development of cognitive impairment. PND poses a serious threat to the health and quality of life of elderly individuals, leading to prolonged hospitalization, increased patient mortality, and a heightened societal burden. Moreover, specific treatments for PND are lacking, and drug treatments like dexamethasone have shown effectiveness in improving postoperative cognitive impairment, alongside non-drug interventions such as sleep management, cognitive training, music therapy, close nursing care, monitoring, and traditional Chinese medicine available [14,15,16]. Therefore, further research into the pathogenesis of PND is crucial for developing effective prevention and treatment strategies.
Microbiome research has gained significant attention in recent years, highlighting the intricate connection between microbial composition and human health. This relationship is characterized by direct microbial-host interactions, the modulation of immune and inflammatory responses, the influence of microorganisms and their metabolites on cellular signaling pathways, and potential genotoxic effects [17]. Studies have indicated strong correlations between the composition of the gut microbiota (GM) and both the nervous system [18] and psychosomatic health [19, 20]. The GM plays a crucial role in fermenting dietary fiber to produce short-chain fatty acids (SCFAs), which not only provide energy to the intestinal mucosa but also promote intestinal peristalsis for waste elimination [21]. Disruption of the GM can compromise the intestinal mucosal barrier, leading to the translocation of toxins and metabolites into the bloodstream and triggering inflammatory responses that may impact the central nervous system [22]. Recent research has demonstrated that surgical and anesthetic procedures can alter the GM composition [23,24,25], hypothetically contributing to the development of PND. Thus, analyzing perioperative changes in the intestinal flora and exploring interventions based on these alterations could offer a promising approach to preventing and managing neurocognitive disorders. This narrative review aimed to explore the relationship between the gut microbiome, its metabolic products, and neurocognitive impairment. The focus is on understanding how changes in the gut microbial ecosystem contribute to cognitive deficits. Specifically, we highlight the impact of perioperative gut microbiome dysbiosis on neurocognitive complications. Based on current research, we suggest that manipulating the gut microbiome could be a potential treatment for perioperative neurocognitive disorders.
Methods
A thorough literature search was conducted across PubMed, Web of Science, and CNKI databases for studies up to May 2024. The search utilized key terms related to postoperative cognitive dysfunction, perioperative neurocognitive disorders, cognitive impairment, and the gut microbiome. Both human and animal studies were considered, with initial screening done by J H and TS Q and final selections made by Y B and WJ R. Reference lists of relevant studies were also reviewed for additional publications. Due to the diverse nature of study populations, species, and outcome measures, a narrative review approach was chosen over a systematic one. Inclusion criteria focused on studies exploring the link between neurocognitive impairment and the gut microbiome, with accessibility to full-text articles being a key factor. Exclusion criteria encompassed non-English or non-Chinese publications, correspondence, conference proceedings, and retracted studies.
Results
Characterization of the Gut Microbiota in Patients with Cognition Disorders
Numerous studies have shown notable differences in GM composition between individuals with and without cognitive impairment. Kaiyrlykyzy et al. [26] reported that patients with Alzheimer’s disease (AD) had greater abundances of Acidobacteriota, Verrucomicrobiota, Synergistota, and Planctomycetota, among others, than healthy individuals. Additionally, the Prevotella, Alloprevotella, and Ruminococcus genera were more abundant, while the abundances of Bifidobacterium, Clostridia bacterium, and Lactiplantibacillus were significantly reduced. Bairamian et al. [27] reported that patients with AD exhibited increases in proinflammatory flora (e.g., Bacteroidetes) and decreases in anti-inflammatory flora (e.g., the Firmicutes phylum), revealing potential variations based on geography and population. Zhang and other scholars [28] have conducted preclinical experiments similar to those in previous studies. They discovered that the intestinal microbiota of aged mice with cognitive dysfunction postsurgery differed significantly from that of normal mice. Specifically, certain genera of Bacteroidetes showed notable increases, while bacterial genera such as Lachnospiraceae bacterium A2 and Blautia decreased significantly. Metabolomic analysis revealed decreasing trends in metabolites such as thiamine and long-chain unsaturated fatty acids. It is worth noting that the declining genera are responsible for producing metabolites such as SCFAs, which are beneficial for host health. This suggests that the metabolites of the gut flora play a crucial role in maintaining overall health. A recent study from Korea [29] revealed that patients with dementia who underwent fecal microbial transplants exhibited increased levels of Bacteroides, Alistipes, and Odoribacter, with a decrease in Enterococcaceae abundance. Additionally, changes in the expression levels of lipid metabolism genes were observed, potentially contributing to cognitive impairment. These contrasting results underscore the intricate interactions between the host and microbial communities and their combined effects on disease development. The human microbiota exhibits functional redundancy, meaning that changes in a specific strain may not always lead to drastic alterations in physiological function. Moreover, various strains of bacteria within the same genus can interact with the human body in diverse ways. Thus, investigating the impact of the microbiota on human genes and metabolism appears to be more crucial than solely focusing on microbiota changes. Additionally, variations in host genetics and metabolism can influence how the microbiota responds when similar diseases affect different individuals. Overall, patients with cognition disorders may have a GM that is functionally proinflammatory, characterized by an increase in inflammatory flora and a simultaneous decrease in anti-inflammatory flora (Table 1).
Gut Microorganisms and Their Metabolites Are Closely Related to Body Metabolism
The number of microorganisms in the human body is nearly equal to the number of cells in the body [30], with a significant portion residing in the gut microbiota estimated to be approximately 1014 [31]. GM and its metabolites play a crucial role in human health, impacting various aspects, such as growth, development, nutrition, immunity, and metabolism. Research by Bar et al. [32] indicated that a substantial percentage of plasma metabolites can be linked to the composition of the GM, suggesting a profound influence on substance metabolism in the bloodstream. Metabolites produced by microorganisms that travel from the intestines to the circulation can disrupt normal cellular metabolism and immune function. Additionally, studies by Schirmer et al. [33] revealed a notable association between the expression levels of certain cytokines, such as IL-1β, IL-6, and IFNγ, and specific GM populations, highlighting the close relationship between cytokine production and GM. Liu et al. [34] identified variations in the gut microbiota and serum metabolites among populations at varying altitudes. Their findings highlighted a negative correlation between amino acid metabolism, specifically L-glutamine and L-valine metabolism, and the abundance of Bacteroidetes and a positive correlation with the abundance of Proteobacteria. These findings suggest that GM composition plays a crucial role in an individual’s physiological and metabolic functions. Moreover, research by Richards et al. [35] showed that different intestinal flora can impact colonic epithelial cells, resulting in distinct patterns of gene expression. This indicates that various microorganisms may have differing effects on human gene regulation, with specific microbiota potentially influencing specific gene expression. For example, Fusobacterium nucleatum was found to modulate macrophages through the TNFSF9/TRAF1 signaling pathways [36]. Consequently, alterations in gene expression levels induced by GM can result in variations in downstream protein expression and subsequent biological responses.
Gut Mucosal Barrier Disruption due to Microecological Imbalance Increases Central Nervous System Inflammation
The close relationship between the GM and its metabolites and the central nervous system has led to the development of the concept known as the “gut–brain axis.” Chidambaram et al. [37] discussed how the microbiota can stimulate the production and release of abnormal neurotransmitters, impacting cellular metabolism in the central nervous system through direct interactions with nerves such as the enteric and vagus nerves. The resulting signals from this process reach the brain. Wang et al. [38] suggested that, beyond direct anatomical connections, mechanisms of the gut–brain communication may involve the neuroendocrine–hypothalamic–pituitary axis, the intestinal immune system, neurotransmitters, the intestinal mucosal barrier, and the blood‒brain barrier. Furthermore, microorganisms play roles in generating active metabolites such as SCFAs, valeric acid, dopamine, and extracellular vesicles (EVs) of intestinal microbial origin [39, 40]. These metabolites enter the bloodstream and impact the central nervous system, subsequently influencing host cognition and behavior.
Intestinal Mucosal Barrier and the Blood‒Brain Barrier
The intestinal mucosal barrier consists of several layers, comprising an aqueous membrane layer within the intestinal lumen, a glycocalyx and mucus layer, a tightly connected mucosal epithelial layer, a mucosal lamina propria, and immune proteins and cells [41]. Bacteriostatic peptides abound within the mucus layer [42]. This barrier plays a crucial role in screening microorganisms and their metabolites from entering the circulation [40]. It not only preserves the structural integrity of the intestinal tract and facilitates nutrient absorption but also impedes the access of harmful bacteria and their metabolites to the circulation. Research has indicated that the GM can modulate the expression of choroid plexus genes [43], resulting in the production of SCFAs that augment the expression of tight junction proteins such as ZO-1 and OCLN. These proteins assist in preserving the integrity of the blood‒brain barrier and facilitating the clearance of Aβ proteins by microglia, thus mitigating neuroinflammation [44, 45]. Disruption of the intestinal mucosal barrier leads to heightened permeability, enabling harmful flora and toxic metabolites to enter the circulation, thereby eliciting the body’s immune response [46, 47] and influencing the tight junctions between neuronal cells. This disruption indirectly affects the permeability of the blood‒brain barrier.
Immune Dysregulation Induced by the Intestinal Flora Accelerates Cognitive Impairment
Neuroinflammation has been identified as a key mechanism contributing to cognitive impairment, with a substantial body of clinical evidence highlighting the significant impact of immune dysfunction on cognitive decline. In their review article, Loh et al. [48] emphasized the role of microglial and astrocyte dysfunction in the progression of various neurodegenerative diseases, such as AD, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease. Guo et al. [49] further discussed the differential expression of immune-related genes, such as CD177 and S100A12, in patients with AD compared to those with mild cognitive dysfunction, highlighting their crucial roles in neutrophil activation. Additionally, Zang et al. [50] demonstrated that altered gene expression related to centrocyte activation and interferon synthesis can impact cognitive abilities, particularly in depressed patients. The significance of immune factors in cognitive disorders is evident, with recent research emphasizing the role of the gut flora in maintaining immune balance. Early colonization by gut microbes influences immune cell development [51], with long-lasting effects on host health [52]. In the central nervous system, microglia and astrocytes play vital roles as immune cells, and their dysfunction leads to neuroinflammation and negative effects on overall health. Microglia help reduce neuroinflammation by phagocytosing proteins such as Aβ, tau, and α-synuclein [48], while astrocytes are involved in neurotransmitter regulation, signaling, and inflammatory responses within the brain [53]. In animal experiments, it has been observed that dysregulation of the gut microecology can lead to the abnormal activation of microglia and a decrease in synaptic plasticity [54, 55]. This relationship indicates that gut microbes may impact the function of microglia and astrocytes and that the malfunction of these cells can worsen neuroinflammation and result in cognitive impairments.
Microecological Dysregulation Causes Neurotransmitter Abnormalities in the Somatic Circulation
Neurotransmitters play a crucial role in the neurological functioning of the body and are essential for normal physiological metabolism. Abnormalities in certain neurotransmitters can lead to declines in learning and memory, while some abnormalities can contribute to neuroinflammation [56]. Recent research has suggested a connection between the gut microbiota and neurotransmitter production and release. Lynch and Hsiao [57] investigated how the microbiota can activate inactive neurotransmitters and synthesize or degrade neurotransmitters through biotransformation. Konstanti and colleagues [58] demonstrated that Akkermansia muciniphila can produce glutamic acid decarboxylase, which helps in the synthesis of γ-aminobutyric acid, leading to the inhibition of neuroexcitation. Furthermore, microbiota-derived metabolites play a role in host substance metabolism. For instance, Palepu et al. [59] reported in a preclinical study that SCFAs produced by the intestinal flora from the breakdown of dietary fibers can regulate the metabolism of substances such as tryptophan and neurotransmitters. Imbalances in the intestinal microbiota can alter the production and release of neurotransmitters through changes in these derived metabolites. Wang et al. [60] reported that exposure to methylmercury led to notable alterations in the diversity and composition of the intestinal microbiota in mice, resulting in dysbiosis that influenced the release of neurotransmitters such as 5-HT and dopamine into the bloodstream. As a result, it is plausible that irregular metabolism triggered either directly or indirectly by gut microbes could play a role in the onset of cognitive dysfunction.
Dysbiosis of the Intestinal Microbiota Alters EVs Originating from the Microbiome
EVs of bacterial origin serve as a crucial medium for communication among different bacterial colonies and between colonies and hosts. These vesicles primarily consist of lipopolysaccharides, lipids, and proteins [61]. They play significant roles in maintaining the integrity of the host’s intestinal mucosal barrier, supporting the normal function of the host’s immune system, and regulating substance metabolism [62, 63]. Research by Zhai et al. [40] suggests that gut microbes might not be the primary source of circulating microbial DNA, while EVs derived from the GM could have a substantial impact on host health [64, 65]. Variations in flora result in distinct EVs, and dysbiosis could lead to abnormalities in these vesicles, consequently affecting the host differently. Lee and colleagues [66] demonstrated in a preclinical study that transferring fecal microorganisms from elderly mice to young mice increased the likelihood of cognitive deficits, with EVs originating from Paenalcaligenes hominis causing hippocampal damage. Furthermore, Wei et al. [67] reported that EVs from the gut microbiota of AD patients activated GSK-3β proteins, induced tau protein phosphorylation, and enhanced the secretion of inflammatory cytokines in the hippocampus.
Gut Flora Dysbiosis Impairs Autophagy in Nerve Cells
Autophagy is a protective physiological process that cells undergo in response to stressful conditions, serving as a regulatory mechanism to eliminate unwanted substances and abnormal proteins [37]. Impaired autophagy can lead to inflammatory reactions and cellular dysfunction. Research by Cho et al. [68] showed that dysbiosis of the gut microbiota can hinder autophagy in neuronal cells, with reduced blood butyrate levels in mice with dysbiosis leading to increased CASP3 cleavage protein density and deficits in mitochondrial autophagy. However, supplementation with butyrate was found to improve cognitive deficits in mice. Furthermore, studies by Liu et al. [69] demonstrated that the gut flora and its metabolites play a role in influencing neurological autophagy, impacting the progression of Parkinson’s disease. Interestingly, changes in autophagy in intestinal mucosal cells can also alter the intestinal microecology, resulting in increased intestinal inflammation and systemic immune function alterations [70].
Anesthesia Interferes with Gut Microecology and Increases the Risk of Postoperative Neurocognitive Impairment
Surgical procedures have a significant impact on the microbiota, with factors such as anesthetic drugs, type and duration of surgery, surgical site, and postoperative medications influencing the diversity of the intestinal flora. Studies by Liu et al. [71] and Wetzel et al. [72] have shown changes in gut microbial composition following surgeries such as sleeve gastrectomy and procedures for treating Crohn’s disease, respectively. These changes were linked to alterations in lipid metabolism and clinical outcomes. Notably, postoperative cognitive deficits may be associated with an increase in proinflammatory bacteria, similar to findings in hosts with cognitive deficits. Furthermore, surgical-induced intestinal mucosal injury can compromise barrier function, allowing toxic metabolites and bacteria to enter the circulation [73, 74].
Anesthesia Interferes with IntestinalMicroecology
Anesthetic drugs and anesthesia can disrupt the balance of the gut microbiota through direct effects on the microbiota, potentially leading to dysbiosis. Research by Liu et al. [75] suggested that anesthetic drugs have the ability to directly impact the microbiota, potentially causing its death or altering its phenotype. Additionally, the impact of different anesthetic drugs and methods of anesthesia on the microbiota can vary. For instance, a study by Liu et al. [76] compared the effects of sevoflurane, propofol, and a combination of sevoflurane and propofol on postoperative intestinal flora, revealing differences in microbial composition between the groups. Furthermore, the effects of surgical procedures and locations on the intestinal flora can also be significant. In a meta-analysis conducted by Xu et al. [77], the intestinal microbiota of individuals who underwent cholecystectomy exhibited significant differences in 12 species, such as Escherichia–Shigella, Megamonas, Prevotella 9, and Ruminococcus gnavus, compared to healthy individuals. In a separate study, Zhang et al. [78] reported that patients who underwent surgery for ventricular septal defect with cardiopulmonary bypass surgery experienced a decrease in the diversity of the intestinal flora during the postoperative period. The abundance of individual bacterial species also underwent significant changes, with a greater abundance of Enterococci observed in patients with gastrointestinal dysfunction postoperatively than in those without gastrointestinal dysfunction, while the abundance of Bifidobacterium decreased notably. These findings suggest that the impact of surgery on the postoperative intestinal environment varies depending on the surgical site and that interactions between the host and the microbiota lead to alterations in the flora composition.
Opioids can have a notable impact on the GM, leading to disruptions in both microbial and host metabolism. Kolli et al. [79] observed significant increases in the abundances of eight genera, including Parasutterella excrementihominis, in the intestinal tracts of mice fed morphine, while the abundance of Lactobacillus johnsonii decreased. Wang et al. [80] reported a decrease in the alpha diversity of the intestinal microbiota and a notable increase in potentially pathogenic intestinal flora in mice after morphine administration. The representative genera identified in this study included Flavobacterium, Enterococcus, Fusobacterium, Sutterella, and Clostridioides.
Surgical stress significantly affects the body’s physiology and metabolism, impacting the host’s stress response to surgery. It also leads to alterations in the body’s microecology, which can greatly influence the recovery and prognosis of postoperative patients.
Dysregulation of the Microbiota After Anesthetic Surgery Promotes Postoperative Neurocognitive Deficits
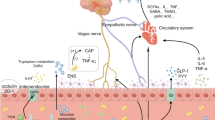
Surgery, while effective in treating many diseases, can also lead to cognitive impairment. The exact mechanisms underlying this phenomenon remain unclear, but potential causes include neuroinflammation and neuronal damage induced by anesthetic drugs during surgery [81], dysregulation of the microecological environment, autophagy deficits in neuronal cells due to surgical and anesthesia-related factors, and immune dysregulation during and after surgery. However, the neurotoxic effects of anesthetic agents remain controversial. Devroe et al. [82] found that sevoflurane anesthesia is an independent risk factor for postoperative delirium in children. Interestingly, Davidson et al. [83] reported that brief exposure to sevoflurane does not affect neurodevelopment and cognitive changes in children. Future studies with larger sample sizes and diverse populations are needed to further investigate this issue. Research has shown that abnormal metabolites in the blood of individuals with dysbiosis of the gut flora can worsen cognitive impairment. For example [84], studies have demonstrated that increased levels of VPA in the blood of mice with postoperative cognitive dysfunction can lead to inflammatory responses in the nervous system and impair learning and memory. Strategies such as exercise and fecal microbial transplantation have been found to reduce blood VPA levels and improve postoperative cognitive function. Furthermore, surgery-induced dysbiosis of the gut flora can affect the ability of EVs to enter the bloodstream [85], with EVs from different flora sources exerting varying effects on the host [86]. Changes in the gut flora composition after surgery have been linked to alterations in the expression levels of proteins involved in maintaining blood‒brain barrier integrity. Treatment with cefazolin has been shown to increase the abundance of bacteria that produce SCFAs in the gut, leading to elevated SCFA levels and partial reversal of postoperative cognitive decline [87] (Fig. 1).
The role of intestinal flora and their derivatives in perioperative neurocognitive dysfunction (by Figdraw). Anesthetic surgical stress and opioid use disrupt the intestinal microecology, leading to an increase in pro-inflammatory flora. This disruption can result in the breakdown of the intestinal mucosal barrier, heightened intestinal permeability, and the circulation of intestinal flora and toxic metabolites, triggering an immune response. This immune response may disrupt the blood‒brain barrier and cause damage to neuronal cells. Additionally, microecological dysregulation can enhance the entry of abnormal cell membrane vesicles into the circulation, promoting neuroinflammatory responses. These combined effects ultimately contribute to the development of neurocognitive deficits
Personalized Regulation of the Gut Microflora May Be a New Direction for Treating Cognitive Disorders
Research efforts have focused primarily on regulating the intestinal flora and improving diets to prevent and control disease development. However, the selection of specific probiotics or probiotic combinations for various diseases remains a key area requiring further investigation. The evidence suggests the potential for regulating dysbiosis. For instance, Ren et al. [24] demonstrated in a preclinical trial that modifying the intestinal flora through dietary changes led to positive outcomes, potentially due to repairing intestinal flora disorders, reducing neuroinflammation, and enhancing nerve function. Similarly, Bosch et al. [88] reported that the administration of GV-971 to mice altered the intestinal flora, reduced Aβ protein deposition, alleviated neuroinflammation, and improved neurocognitive deficits by targeting the microbiota–microglia–amyloid axis. A study from Korea [89] confirmed the role of probiotics in liver function protection, showing that consuming beverages with L. holzapfelii preparations restored the intestinal flora structure and decreased serum transaminase levels. Notably, the effectiveness of a single probiotic species may be limited, and the same flora may not have consistent effects across different organs and systems. For example, a study by Johnstone and colleagues [90] revealed no significant difference in therapeutic efficacy between Lactobacillus rhamnosus GG and a placebo in improving respiratory-associated pneumonia. Lactobacillus rhamnosus GG showed no significant difference in therapeutic effect compared to the placebo. Further research studies are required to better understand the role of gut flora in various groups and diseases.
Summary
In conclusion, dysregulation of the gut microbiota induces PND. Direct interactions between the microbiota and the host, where microbial metabolites stimulate the host to produce an immune-inflammatory response, may be the key mechanism in this induction. This process involves the disruption of gut mucosal barrier function and increased permeability of the blood‒brain barrier, allowing toxic metabolites and abnormal extracellular vesicles to enter the body’s circulation. Surgery and anesthesia can disrupt the intestinal microecology, leading to an ecological imbalance that may initiate PND. Therefore, implementing appropriate preoperative and postoperative interventions to minimize damage to the microbiota ecosystem and repair microecological disturbances could be promising therapeutic strategies. Further clinical and experimental studies are necessary to provide new evidence for standardizing the protection and adjustment of the intestinal flora, as well as identifying specific therapeutic targets for the standardized management of PND.
Data Availability
No datasets were generated or analyzed during the current study.
References
Dilmen OK, Meco BC, Evered LA, Radtke FM (2024) Postoperative neurocognitive disorders: a clinical guide. J Clin Anesth 92:111320. https://doi.org/10.1016/j.jclinane.2023.111320
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS et al (2018) Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesth Analg 127(5):1189–1195. https://doi.org/10.1213/ane.0000000000003634
Pan CC, Chu CS, Chen CL, Chuang YC, Chen NC (2021) Factors affecting rapid cognitive decline in patients with Alzheimer's disease: a longitudinal follow-up study. Int J Environ Res Public Health 18(16); https://doi.org/10.3390/ijerph18168576.
Bhushan S, Li Y, Huang X, Cheng H, Gao K, Xiao Z (2021) Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: a review article. Int J Surg 95:106163. https://doi.org/10.1016/j.ijsu.2021.106163
Silva AR, Regueira P, Albuquerque E, Baldeiras I, Cardoso AL, Santana I, Cerejeira J (2021) Estimates of geriatric delirium frequency in noncardiac surgeries and its evaluation across the years: a systematic review and meta-analysis. J Am Med Dir Assoc 22(3):613–20.e9. https://doi.org/10.1016/j.jamda.2020.08.017
Oriby ME, Elrashidy AA, Elsharkawy A, Ahmed SA (2023) Effects of ketamine or dexmedetomidine on postoperative cognitive dysfunction after cataract surgery: a randomized controlled trial. Indian J Anaesth 67(2):186–193. https://doi.org/10.4103/ija.ija_429_22
Kuzminskaite V, Kontrimaviciute E, Kauzonas E, Slauzgalvyte I, Bukelyte G, Bruzyte-Narkiene G, Jatuzis D (2023) Sevoflurane and desflurane effects on early cognitive function after low-risk surgery: a randomized clinical trial. Brain Behav 13(6):e3017. https://doi.org/10.1002/brb3.3017
Deng H, Wu Y, Gao P, Kong D, Pan C, Xu S et al (2023) Preoperative pain facilitates postoperative cognitive dysfunction via periaqueductal gray matter-dorsal raphe circuit. Neuroscience 524:209–219. https://doi.org/10.1016/j.neuroscience.2023.03.019
Ren S, Yuan F, Yuan S, Zang C, Zhang Y, Lang B (2022) Early cognitive dysfunction in elderly patients after total knee arthroplasty: an analysis of risk factors and cognitive functional levels. Biomed Res Int 2022:5372603. https://doi.org/10.1155/2022/5372603
Ding X, Gao X, Wang Z, Jiang X, Lu S, Xu J et al (2021) Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J Mol Neurosci 71(3):515–526. https://doi.org/10.1007/s12031-020-01673-x
Zhao Q, Wan H, Pan H, Xu Y (2024) Postoperative cognitive dysfunction-current research progress. Front Behav Neurosci 18:1328790. https://doi.org/10.3389/fnbeh.2024.1328790
Somnuke P, Srishewachart P, Jiraphorncharas C, Khempetch A, Weeranithan J, Suraarunsumrit P et al (2024) Early postoperative neurocognitive complications in elderly patients: comparing those with and without preexisting mild cognitive impairment- a prospective study. BMC Geriatr 24(1):84. https://doi.org/10.1186/s12877-024-04663-5
Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N (2017) Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol 34(11):776–784. https://doi.org/10.1097/eja.0000000000000647
Kong H, Xu LM, Wang DX (2022) Perioperative neurocognitive disorders: a narrative review focusing on diagnosis, prevention, and treatment. CNS Neurosci Ther 28(8):1147–1167. https://doi.org/10.1111/cns.13873
Zhang J, Cairen Z, Shi L, Zhang M, Yang M, Wang Y, Lu Z (2023) Acupuncture-related techniques for postoperative cognitive complications: a systemic review and meta-analysis. Perioper Med (Lond) 12(1):14. https://doi.org/10.1186/s13741-023-00303-5
Zhao L, Guo Y, Zhou X, Mao W, Zhu H, Chen L et al (2024) The research progress of perioperative non-pharmacological interventions on postoperative cognitive dysfunction: a narrative review. Front Neurol 15:1369821. https://doi.org/10.3389/fneur.2024.1369821
Bhatt AP, Redinbo MR, Bultman SJ (2017) The role of the microbiome in cancer development and therapy. CA Cancer J Clin 67(4):326–344. https://doi.org/10.3322/caac.21398
Zhu F, Zhao Y, Arnold DL, Bar-Or A, Bernstein CN, Bonner C et al (2024) A cross-sectional study of MRI features and the gut microbiome in pediatric-onset multiple sclerosis. Ann Clin Transl Neurol 11(2):486–496. https://doi.org/10.1002/acn3.51970
Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM (2021) The gut microbiota in anxiety and depression - a systematic review. Clin Psychol Rev 83:101943. https://doi.org/10.1016/j.cpr.2020.101943
Zhou M, Fan Y, Xu L, Yu Z, Wang S, Xu H et al (2023) Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 11(1):145. https://doi.org/10.1186/s40168-023-01589-9
Wu Y, Jha R, Li A, Liu H, Zhang Z, Zhang C et al (2022) Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol Spectr 10(6):e0165122. https://doi.org/10.1128/spectrum.01651-22
Fang Z, Shen G, Amin N, Lou C, Wang C, Fang M (2023) Effects of neuroinflammation and autophagy on the structure of the blood-brain barrier in ADHD model. Neuroscience 530:17–25. https://doi.org/10.1016/j.neuroscience.2023.08.025
Zhang L, Cheng X, Xia L, Liu N, Liu L, Liu S et al (2024) Analysis of 16s rRNA gene sequencing in feces: the impact of bariatric surgery on the gut microbiota in patients with obesity. Obes Surg. https://doi.org/10.1007/s11695-024-07087-7
Ren L, Liang H, Zhu L, Yang X, Zhang H, Sun N et al (2024) Dietary restriction improves perioperative neurocognitive disorders by inhibiting neuroinflammation and gut microbial dysbiosis. Neuroscience 540:48–67. https://doi.org/10.1016/j.neuroscience.2024.01.012
Tsigalou C, Paraschaki A, Bragazzi NL, Aftzoglou K, Bezirtzoglou E, Tsakris Z et al (2023) Alterations of gut microbiome following gastrointestinal surgical procedures and their potential complications. Front Cell Infect Microbiol 13:1191126. https://doi.org/10.3389/fcimb.2023.1191126
Kaiyrlykyzy A, Kozhakhmetov S, Babenko D, Zholdasbekova G, Alzhanova D, Olzhayev F et al (2022) Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci Rep 12(1):15115. https://doi.org/10.1038/s41598-022-19393-0
Bairamian D, Sha S, Rolhion N, Sokol H, Dorothée G, Lemere CA, Krantic S (2022) Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol Neurodegener 17(1):19. https://doi.org/10.1186/s13024-022-00522-2
Zhang Sh, Jia Xy, Wu Q, Jin J, Xu Ls, Yang L et al (2023) The involvement of the gut microbiota in postoperative cognitive dysfunction based on integrated metagenomic and metabolomics analysis. Microbiol Spectr 11(6):e0310423; https://doi.org/10.1128/spectrum.03104-23.
Kim JS, Park H, Lee JH, Shin J, Cha B, Kwon KS et al (2024) Effect of altered gene expression in lipid metabolism on cognitive improvement in patients with Alzheimer’s dementia following fecal microbiota transplantation: a preliminary study. Ther Adv Neurol Disord 17:17562864231218180. https://doi.org/10.1177/17562864231218181
Walker AW, Hoyles L (2023) Human microbiome myths and misconceptions. Nat Microbiol 8(8):1392–1396. https://doi.org/10.1038/s41564-023-01426-7
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14(8):e1002533. https://doi.org/10.1371/journal.pbio.1002533
Bar N, Korem T, Weissbrod O, Zeevi D, Rothschild D, Leviatan S et al (2020) A reference map of potential determinants for the human serum metabolome. Nature 588(7836):135–140. https://doi.org/10.1038/s41586-020-2896-2
Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA et al (2016) Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167(4):1125–36.e8. https://doi.org/10.1016/j.cell.2016.10.020
Liu D, Gao X, Huang X, Fan Y, Wang YE, Zhang Y et al (2023) Moderate altitude exposure impacts host fasting blood glucose and serum metabolome by regulation of the intestinal flora. Sci Total Environ 905:167016. https://doi.org/10.1016/j.scitotenv.2023.167016
Richards AL, Muehlbauer AL, Alazizi A, Burns MB, Findley A, Messina F et al (2019) Gut microbiota has a widespread and modifiable effect on host gene regulation. mSystems. 4(5); https://doi.org/10.1128/mSystems.00323-18.
Zou S, Yang C, Zhang J, Zhong D, Meng M, Zhang L et al (2024) Multi-omic profiling reveals associations between the gut microbiome, host genome and transcriptome in patients with colorectal cancer. J Transl Med 22(1):175. https://doi.org/10.1186/s12967-024-04984-4
Chidambaram SB, Essa MM, Rathipriya AG, Bishir M, Ray B, Mahalakshmi AM et al (2022) Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacol Ther 231:107988. https://doi.org/10.1016/j.pharmthera.2021.107988
Wang HX, Wang YP (2016) Gut Microbiota-brain Axis. Chin Med J (Engl) 129(19):2373–2380. https://doi.org/10.4103/0366-6999.190667
Mhanna A, Martini N, Hmaydoosh G, Hamwi G, Jarjanazi M, Zaifah G et al (2024) The correlation between gut microbiota and both neurotransmitters and mental disorders: a narrative review. Medicine (Baltimore) 103(5):e37114. https://doi.org/10.1097/md.0000000000037114
Zhai T, Ren W, Ji X, Wang Y, Chen H, Jin Y et al (2024) Distinct compositions and functions of circulating microbial DNA in the peripheral blood compared to fecal microbial DNA in healthy individuals. mSystems e0000824; https://doi.org/10.1128/msystems.00008-24.
Camilleri M (2021) Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin Transl Gastroenterol 12(1):e00308. https://doi.org/10.14309/ctg.0000000000000308
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A et al (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7(10):e196. https://doi.org/10.1038/ctg.2016.54
Knox EG, Lynch CMK, Lee YS, O’Driscoll CM, Clarke G, Cryan JF, Aburto MR (2023) The gut microbiota is important for the maintenance of blood-cerebrospinal fluid barrier integrity. Eur J Neurosci 57(2):233–241. https://doi.org/10.1111/ejn.15878
Li Z, Zhang F, Sun M, Liu J, Zhao L, Liu S et al (2023) The modulatory effects of gut microbes and metabolites on blood-brain barrier integrity and brain function in sepsis-associated encephalopathy. PeerJ 11:e15122. https://doi.org/10.7717/peerj.15122
Xie J, Bruggeman A, De Nolf C, Vandendriessche C, Van Imschoot G, Van Wonterghem E et al (2023) Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. Embo j 42(17):e111515. https://doi.org/10.15252/embj.2022111515
Brown JA, Codreanu SG, Shi M, Sherrod SD, Markov DA, Neely MD et al (2016) Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J Neuroinflammation 13(1):306. https://doi.org/10.1186/s12974-016-0760-y
Gong X, Ma Y, Deng X, Li A, Li X, Kong X et al (2024) Intestinal dysbiosis exacerbates susceptibility to the anti-NMDA receptor encephalitis-like phenotype by changing blood brain barrier permeability and immune homeostasis. Brain Behav Immun 116:34–51. https://doi.org/10.1016/j.bbi.2023.11.030
Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH et al (2024) Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther 9(1):37. https://doi.org/10.1038/s41392-024-01743-1
Guo Z, Peng X, Li HY, Wang Y, Qian Y, Wang Z et al (2019) Evaluation of peripheral immune dysregulation in Alzheimer’s disease and vascular dementia. J Alzheimers Dis 71(4):1175–1186. https://doi.org/10.3233/jad-190666
Zang JCS, Hohoff C, Van Assche E, Lange P, Kraft M, Sandmann S et al (2023) Immune gene co-expression signatures implicated in occurence and persistence of cognitive dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 127:110826. https://doi.org/10.1016/j.pnpbp.2023.110826
Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu WH, Saldana-Morales FB et al (2021) Thymic development of gut-microbiota-specific T cells. Nature 594(7863):413–417. https://doi.org/10.1038/s41586-021-03531-1
Ver Heul A, Planer J, Kau AL (2019) The human microbiota and asthma. Clin Rev Allergy Immunol 57(3):350–363. https://doi.org/10.1007/s12016-018-8719-7
Syvänen V, Koistinaho J, Lehtonen Š (2024) Identification of the abnormalities in astrocytic functions as potential drug targets for neurodegenerative disease. Expert Opin Drug Discov 1–14; https://doi.org/10.1080/17460441.2024.2322988.
He H, He H, Mo L, Yuan Q, Xiao C, Ma Q et al (2024) Gut microbiota regulate stress resistance by influencing microglia-neuron interactions in the hippocampus. Brain Behav Immun Health 36:100729. https://doi.org/10.1016/j.bbih.2024.100729
He H, He H, Mo L, You Z, Zhang J (2024) Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav Immun 115:280–294. https://doi.org/10.1016/j.bbi.2023.10.031
Quan W, Qiao CM, Niu GY, Wu J, Zhao LP, Cui C et al (2023) Trimethylamine N-oxide exacerbates neuroinflammation and motor dysfunction in an acute MPTP mice model of Parkinson's disease. Brain Sci 13(5); https://doi.org/10.3390/brainsci13050790.
Lynch JB, Hsiao EY (2023) Toward understanding links between the microbiome and neurotransmitters. Ann N Y Acad Sci 1524(1):10–16. https://doi.org/10.1111/nyas.14993
Konstanti P, Ligthart K, Fryganas C, Constantinos P, Smidt H, de Vos WM, Belzer C (2024) Physiology of γ-aminobutyric acid production by Akkermansia muciniphila. Appl Environ Microbiol 90(1):e0112123. https://doi.org/10.1128/aem.01121-23
Palepu MSK, Gajula SNR, K M, Sonti R, Dandekar MP (2024) SCFAs supplementation rescues anxiety- and depression-like phenotypes generated by fecal engraftment of treatment-resistant depression rats. ACS Chem Neurosci https://doi.org/10.1021/acschemneuro.3c00727.
Wang W, Chen F, Zhang L, Wen F, Yu Q, Li P, Zhang A (2023) Neurotransmitter disturbances caused by methylmercury exposure: microbiota-gut-brain interaction. Sci Total Environ 873:162358. https://doi.org/10.1016/j.scitotenv.2023.162358
Díaz-Garrido N, Badia J, Baldomà L (2021) Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles 10(13):e12161. https://doi.org/10.1002/jev2.12161
Díez-Sainz E, Milagro FI, Riezu-Boj JI, Lorente-Cebrián S (2022) Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J Physiol Biochem 78(2):485–499. https://doi.org/10.1007/s13105-021-00837-6
Tan J, Ni D, Taitz J, Pinget GV, Read M, Senior A et al (2022) Dietary protein increases T-cell-independent sIgA production through changes in gut microbiota-derived extracellular vesicles. Nat Commun 13(1):4336. https://doi.org/10.1038/s41467-022-31761-y
Macia L, Nanan R, Hosseini-Beheshti E, Grau GE (2019) Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci 21(1); https://doi.org/10.3390/ijms21010107.
Schaack B, Hindré T, Quansah N, Hannani D, Mercier C, Laurin D (2022) Microbiota-derived extracellular vesicles detected in human blood from healthy donors. Int J Mol Sci 23(22); https://doi.org/10.3390/ijms232213787.
Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, Kim DH (2020) The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 8(1):107. https://doi.org/10.1186/s40168-020-00881-2
Wei S, Peng W, Mai Y, Li K, Wei W, Hu L et al (2020) Outer membrane vesicles enhance tau phosphorylation and contribute to cognitive impairment. J Cell Physiol 235(5):4843–4855. https://doi.org/10.1002/jcp.29362
Cho JH, Chae CW, Lim JR, Jung YH, Han SJ, Yoon JH et al (2024) Sodium butyrate ameliorates high glucose-suppressed neuronal mitophagy by restoring PRKN expression via inhibiting the RELA-HDAC8 complex. Autophagy 1–18; https://doi.org/10.1080/15548627.2024.2323785.
Liu X, Du ZR, Wang X, Luk KH, Chan CH, Cao X et al (2021) Colonic dopaminergic neurons changed reversely with those in the midbrain via gut microbiota-mediated autophagy in a chronic Parkinson’s disease mice model. Front Aging Neurosci 13:649627. https://doi.org/10.3389/fnagi.2021.649627
Zhang P, Holowatyj AN, Ulrich CM, Edgar BA (2019) Tumor suppressive autophagy in intestinal stem cells controls gut homeostasis. Autophagy 15(9):1668–1670. https://doi.org/10.1080/15548627.2019.1633863
Liu C, Xu Q, Dong S, Ding H, Li B, Zhang D et al (2024) New mechanistic insights of anti-obesity by sleeve gastrectomy-altered gut microbiota and lipid metabolism. Front Endocrinol (Lausanne) 15:1338147. https://doi.org/10.3389/fendo.2024.1338147
Wetzel S, Müller A, Kohnert E, Mehrbarzin N, Huber R, Häcker G et al (2023) Longitudinal dynamics of gut bacteriome and mycobiome interactions pre- and post-visceral surgery in Crohn’s disease. Front Cell Infect Microbiol 13:1275405. https://doi.org/10.3389/fcimb.2023.1275405
Yang J, He Y, Liao X, Hu J, Li K (2023) Does postoperative pulmonary infection correlate with intestinal flora following gastric cancer surgery? - a nested case-control study. Front Microbiol 14:1267750. https://doi.org/10.3389/fmicb.2023.1267750
Pan C, Zhang H, Zhang L, Chen L, Xu L, Xu N et al (2023) Surgery-induced gut microbial dysbiosis promotes cognitive impairment via regulation of intestinal function and the metabolite palmitic amide. Microbiome 11(1):248. https://doi.org/10.1186/s40168-023-01689-6
Liu L, Shang L, Jin D, Wu X, Long B (2022) General anesthesia bullies the gut: a toxic relationship with dysbiosis and cognitive dysfunction. Psychopharmacology 239(3):709–728. https://doi.org/10.1007/s00213-022-06096-7
Liu H, Qu X, Yin X, Li J, Cao Y, Wang Y et al (2022) Intestinal microbiome and metabolome changes induced by sevoflurane, propofol, and sevoflurane-propofol anaesthesia in patients undergoing nephrectomy. Br J Anaesth 129(2):e38–e40. https://doi.org/10.1016/j.bja.2022.04.028
Xu F, Chen R, Zhang C, Wang H, Ding Z, Yu L et al (2023) Cholecystectomy significantly alters gut microbiota homeostasis and metabolic profiles: a cross-sectional study. Nutrients 15(20); https://doi.org/10.3390/nu15204399.
Zhang QL, Zhou SJ, Chen XH, Chen Q (2024) Changes of intestinal flora and the effect on intestinal function in infants with ventricular septal defect after cardiopulmonary bypass surgery. Curr Probl Cardiol 49(1 Pt B):102111. https://doi.org/10.1016/j.cpcardiol.2023.102111
Kolli U, Jalodia R, Moidunny S, Singh PK, Ban Y, Tao J et al (2023) Multi-omics analysis revealing the interplay between gut microbiome and the host following opioid use. Gut Microbes 15(2):2246184. https://doi.org/10.1080/19490976.2023.2246184
Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8(1):3596. https://doi.org/10.1038/s41598-018-21915-8
Miao M, Han Y, Wang Y, Wang J, Zhu R, Yang Y et al (2024) Dysregulation of iron homeostasis and ferroptosis in sevoflurane and isoflurane associated perioperative neurocognitive disorders. CNS Neurosci Ther 30(2):e14553. https://doi.org/10.1111/cns.14553
Devroe S, Devriese L, Debuck F, Fieuws S, Cools B, Gewillig M et al (2020) Effect of xenon and dexmedetomidine as adjuncts for general anesthesia on postoperative emergence delirium after elective cardiac catheterization in children: study protocol for a randomized, controlled, pilot trial. Trials 21(1):310. https://doi.org/10.1186/s13063-020-4231-5
Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G et al (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387(10015):239–250. https://doi.org/10.1016/s0140-6736(15)00608-x
Lai Z, Shan W, Li J, Min J, Zeng X, Zuo Z (2021) Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol Psychiatry 26(12):7167–7187. https://doi.org/10.1038/s41380-021-01291-y
Huh YJ, Seo JY, Nam J, Yang J, McDowell A, Kim YK, Lee JH (2019) Bariatric/metabolic surgery induces noticeable changes of microbiota and their secreting extracellular vesicle composition in the gut. Obes Surg 29(8):2470–2484. https://doi.org/10.1007/s11695-019-03852-1
Diaz-Garrido N, Badia J, Baldomà L (2022) Modulation of dendritic cells by microbiota extracellular vesicles influences the cytokine profile and exosome cargo. Nutrients 14(2); https://doi.org/10.3390/nu14020344.
Luo A, Li S, Wang X, Xie Z, Li S, Hua D (2021) Cefazolin improves anesthesia and surgery-induced cognitive impairments by modulating blood-brain barrier function, gut bacteria and short chain fatty acids. Front Aging Neurosci 13:748637. https://doi.org/10.3389/fnagi.2021.748637
Bosch ME, Dodiya HB, Michalkiewicz J, Lee C, Shaik SM, Weigle IQ et al (2024) Sodium oligomannate alters gut microbiota, reduces cerebral amyloidosis and reactive microglia in a sex-specific manner. Mol Neurodegener 19(1):18. https://doi.org/10.1186/s13024-023-00700-w
Yang J, McDowell A, Kim EK, Seo H, Yum K, Lee WH et al (2019) Consumption of a Leuconostoc holzapfelii-enriched synbiotic beverage alters the composition of the microbiota and microbial extracellular vesicles. Exp Mol Med 51(8):1–11. https://doi.org/10.1038/s12276-019-0288-1
Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J et al (2021) Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA 326(11):1024–1033. https://doi.org/10.1001/jama.2021.13355
Yamashiro K, Takabayashi K, Kamagata K, Nishimoto Y, Togashi Y, Yamauchi Y et al (2024) Free water in gray matter linked to gut microbiota changes with decreased butyrate producers in Alzheimer’s disease and mild cognitive impairment. Neurobiol Dis 193:106464. https://doi.org/10.1016/j.nbd.2024.106464
Laske C, Müller S, Munk MHJ, Honold I, Willmann M, Peter S, Schoppmeier U (2024) Prognostic value of gut microbiome for conversion from mild cognitive impairment to Alzheimer's disease dementia within 4 years: results from the AlzBiom study. Int J Mol Sci 25(3); https://doi.org/10.3390/ijms25031906.
Kim EJ, Kim JS, Park SE, Seo SH, Cho KM, Kwon SJ et al (2023) Association between Mild cognitive impairment and gut microbiota in elderly Korean patients. J Microbiol Biotechnol 33(10):1376–1383. https://doi.org/10.4014/jmb.2305.05009
McLeod A, Penalver Bernabe B, Xia Y, Sanchez-Flack J, Lamar M, Schiffer L et al (2023) Comparing the gut microbiome of obese, African American, older adults with and without mild cognitive impairment. PLoS ONE 18(2):e0280211. https://doi.org/10.1371/journal.pone.0280211
Zhu Z, Ma X, Wu J, Xiao Z, Wu W, Ding S et al (2022) Altered gut microbiota and its clinical relevance in mild cognitive impairment and Alzheimer's disease: Shanghai aging study and Shanghai memory study. Nutrients 14(19); https://doi.org/10.3390/nu14193959.
Khedr EM, Omeran N, Karam-Allah Ramadan H, Ahmed GK, Abdelwarith AM (2022) Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment. J Alzheimers Dis 88(3):1103–1114. https://doi.org/10.3233/jad-220176
Pan Q, Li YQ, Guo K, Xue M, Gan Y, Wang K et al (2021) Elderly patients with mild cognitive impairment exhibit altered gut microbiota profiles. J Immunol Res 2021:5578958. https://doi.org/10.1155/2021/5578958
Lian X, Zhu Q, Sun L, Cheng Y (2021) Effect of anesthesia/surgery on gut microbiota and fecal metabolites and their relationship with cognitive dysfunction. Front Syst Neurosci 15:655695. https://doi.org/10.3389/fnsys.2021.655695
Acknowledgements
Not applicable
Funding
This study was supported by the Key Project of the Basic Research Program of Yunnan Province (No. 202201AS070009), the Talent Project–Famous Doctor Project of Yunnan Province (XDYC-MY-2022–0029), the Foreign Experts Affairs Bureau of Yunnan Province (202305AQ350008), the Project of Basic Research Program of Yunnan Province (Nos. 202301AY070001-079 and 202201AY070001-263), and the Basic Research Plan Project of Yunnan Province (202301AT070094).
Author information
Authors and Affiliations
Contributions
JP, YJL, and YY were involved in the review concept and JP, YJL, WJR, and YY acquired the funding. JH, TSQ, and YB conducted the literature review and wrote the original draft; JH made the figures; WJR made the tables; WJR, YY, JCH, AXM, XDL, YJL, DPT, and RSL made the first revision of the manuscript; and JP finalized the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Qin, TS., Bo, Y. et al. The Role of the Intestinal Flora and Its Derivatives in Neurocognitive Disorders: A Narrative Review from Surgical Perspective. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04322-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04322-1