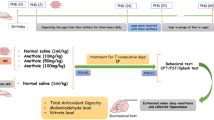
Abstract
Major depressive disorder (MDD) is a severe disorder that causes enormous loss of quality of life, and among the factors underlying MDD is stress in maternal deprivation (MD). In addition, classic pharmacotherapy has presented severe adverse effects. Centella asiatica (C. asiatica) demonstrates a potential neuroprotective effect but has not yet been evaluated in MD models. This study aimed to evaluate the effect of C. asiatica extract and the active compound madecassic acid on possible depressive-like behavior, inflammation, and oxidative stress in the hippocampus and serum of young rats submitted to MD in the first days of life. Rats (after the first day of birth) were separated from the mother for 3 h a day for 10 days. When adults, these animals were divided into groups and submitted to treatment for 14 days. After subjecting the animals to protocols of locomotor activity in the open field and behavioral despair in the forced swimming test, researchers then euthanized the animals. The hippocampus and serum were collected and analyzed for the inflammatory cytokines and oxidative markers. The C. asiatica extract and active compound reversed or reduced depressive-like behaviors, inflammation in the hippocampus, and oxidative stress in serum and hippocampus. These results suggest that C. asiatica and madecassic acid have potential antidepressant action, at least partially, through anti-inflammatory and antioxidant profiles.









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Larsen MH, Mikkelsen JD, Hay-Schmidt A, Sandi C (2010) Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiatr Res 44:808–816. https://doi.org/10.1016/j.jpsychires.2010.01.005
Daskalakis NP, Bagot RC, Parker KJ et al (2013) The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38:1858–1873. https://doi.org/10.1016/j.psyneuen.2013.06.008
Ignácio ZM, Réus GZ, Abelaira HM, Quevedo J (2014) Epigenetic and epistatic interactions between serotonin transporter and brain-derived neurotrophic factor genetic polymorphism: Insights in depression. Neuroscience 275:455–468. https://doi.org/10.1016/j.neuroscience.2014.06.036
Nemeroff CB, Owens MJ (2002) Treatment of mood disorders. Nat Neurosci 5:1068–1070. https://doi.org/10.1038/nn943
Slavich GM, Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140:774–815. https://doi.org/10.1037/a0035302
WHO (2017) Mental health of older adults. https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults. Accessed 23 Dec 2023
Frey A-L, McCabe C (2020) Effects of serotonin and dopamine depletion on neural prediction computations during social learning. Neuropsychopharmacology 45:1431–1437. https://doi.org/10.1038/s41386-020-0678-z
Katz MM, Maas JW, Frazer A et al (1994) Drug-induced actions on brain neurotransmitter systems and changes in the behaviors and emotions of depressed patients. Neuropsychopharmacology 11:89–100. https://doi.org/10.1038/npp.1994.38
Barch DM, Tillman R, Kelly D et al (2019) Hippocampal volume and depression among young children. Psychiatry Res Neuroimaging 288:21–28. https://doi.org/10.1016/j.pscychresns.2019.04.012
Song Z, Shen F, Zhang Z et al (2020) Calpain inhibition ameliorates depression-like behaviors by reducing inflammation and promoting synaptic protein expression in the hippocampus. Neuropharmacology 174:108175. https://doi.org/10.1016/j.neuropharm.2020.108175
Xu Y, Sheng H, Tang Z et al (2015) Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav Brain Res 288:71–78. https://doi.org/10.1016/j.bbr.2015.04.017
Zhou Q-G, Hu Y, Hua Y et al (2007) Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 103:1843–1854. https://doi.org/10.1111/j.1471-4159.2007.04914.x
Jou S-H, Chiu N-Y, Liu C-S (2009) Mitochondrial dysfunction and psychiatric disorders. Chang Gung Med J 32:370–379
Streck EL, Gonçalves CL, Furlanetto CB et al (2014) Mitochondria and the central nervous system: searching for a pathophysiological basis of psychiatric disorders. Rev Bras Psiquiatr 36:156–167. https://doi.org/10.1590/1516-4446-2013-1224
Che Y, Zhou Z, Shu Y et al (2015) Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci Lett 584:208–213. https://doi.org/10.1016/j.neulet.2014.10.031
Garabadu D, Ahmad A, Krishnamurthy S (2015) Risperidone attenuates modified stress–re-stress paradigm-induced mitochondrial dysfunction and apoptosis in rats exhibiting post-traumatic stress disorder-like symptoms. J Mol Neurosci 56:299–312. https://doi.org/10.1007/s12031-015-0532-7
Réus GZ, Carlessi AS, Titus SE et al (2015) A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation: long antidepressant effects of ketamine. Dev Neurobiol 75:1268–1281. https://doi.org/10.1002/dneu.22283
Mokoena ML, Harvey BH, Viljoen F et al (2015) Ozone exposure of Flinders sensitive line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology 232:2921–2938. https://doi.org/10.1007/s00213-015-3928-8
Birben E, Sahiner UM, Sackesen C et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Bakunina N, Pariante CM, Zunszain PA (2015) Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144:365–373. https://doi.org/10.1111/imm.12443
Dinan TG (2009) Inflammatory markers in depression. Curr Opin Psychiatry 22:32–36. https://doi.org/10.1097/YCO.0b013e328315a561
Dowlati Y, Herrmann N, Swardfager W et al (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. https://doi.org/10.1016/j.biopsych.2009.09.033
Williams LM, Debattista C, Duchemin A-M et al (2016) Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 6:e799–e799. https://doi.org/10.1038/tp.2016.61
Zhang Y, Wang Y, Wang L et al (2015) Dopamine receptor D2 and associated microRNAs are involved in stress susceptibility and resistance to escitalopram treatment. Int J Neuropsychopharmacol 18:pyv025–pyv025. https://doi.org/10.1093/ijnp/pyv025
Nishi M (2020) Effects of early-life stress on the brain and behaviors: implications of early maternal separation in rodents. Int J Mol Sci 21:7212. https://doi.org/10.3390/ijms21197212
Ellenbroek BA, Angelucci F, Husum H, Mathé AA (2016) Gene-environment interactions in a rat model of depression. Maternal separation affects neurotensin in selected brain regions. Neuropeptides 59:83–88. https://doi.org/10.1016/j.npep.2016.05.001
Abelaira HM, Rosa T, De Moura AB et al (2022) Combination of electroconvulsive stimulation with ketamine or escitalopram protects the brain against inflammation and oxidative stress induced by maternal deprivation and is critical for associated behaviors in male and female rats. Mol Neurobiol 59:1452–1475. https://doi.org/10.1007/s12035-021-02718-x
Ibanez G, Mercedes BPDC, Vedana KGG, Miasso AI (2014) Adesão e dificuldades relacionadas ao tratamento medicamentoso em pacientes com depressão. Rev Bras Enferm 67:556–562. https://doi.org/10.1590/0034-7167.2014670409
Cunha MDF, Gandini RDC (2009) Adesão e não-adesão ao tratamento farmacológico para depressão. Psicol Teor E Pesqui 25:409–418. https://doi.org/10.1590/S0102-37722009000300015
Marchetti L, Lauria M, Caberlotto L et al (2020) Gene expression signature of antidepressant treatment response/non-response in Flinders sensitive line rats subjected to maternal separation. Eur Neuropsychopharmacol 31:69–85. https://doi.org/10.1016/j.euroneuro.2019.11.004
Souza MSF, Kopittke L (2016) Adesão ao tratamento com psicofármacos: fatores de proteção e motivos de não adesão ao tratamento farmacológico. Rev APS 19:361–369
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661. https://doi.org/10.1021/acs.jnatprod.5b01055
do Prado-Lima PAS, Onsten GA, de Oliveira GN et al (2019) The antidepressant effect of bone marrow mononuclear cell transplantation in chronic stress. J Psychopharmacol (Oxf) 33:632–639. https://doi.org/10.1177/0269881119841562
Jana U, Sur TK, Maity LN et al (2010) A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Med Coll J NMCJ 12:8–11
Lokanathan Y, Omar N, Ahmad Puzi NN et al (2016) Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays J Med Sci MJMS 23:4–14
Lin P-Y, Huang Y-C, Hung C-F (2016) Shortened telomere length in patients with depression: a meta-analytic study. J Psychiatr Res 76:84–93. https://doi.org/10.1016/j.jpsychires.2016.01.015
Wang L, Guo T, Guo Y, Xu Y (2020) Asiaticoside produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice, involving reversion of inflammation and the PKA/pCREB/BDNF signaling pathway. Mol Med Rep 22:2364–2372. https://doi.org/10.3892/mmr.2020.11305
Dutra A (2019) Benefícios da Centella asiatica. Rev Alainura
Park J, Choi J, Son D et al (2017) Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci 18:738. https://doi.org/10.3390/ijms18040738
Kalshetty P, Aswar U, Bodhankar S et al (2012) Antidepressant effects of standardized extract of Centella asiatica L in olfactory bulbectomy model. Biomed Aging Pathol 2:48–53. https://doi.org/10.1016/j.biomag.2012.03.005
Almeida IB, da Silveira Barros Neto JJ, de Oliveira TKB (2016) Princípios básicos de pesquisa com animais de laboratório, 1st edn. IFS, Sergipe
Pałasz A, Suszka-Świtek A, Filipczyk Ł et al (2016) Escitalopram affects spexin expression in the rat hypothalamus, hippocampus and striatum. Pharmacol Rep 68:1326–1331. https://doi.org/10.1016/j.pharep.2016.09.002
Firouzabadi N, Alimoradi N, Najafizadeh M, Najafizadeh P (2021) Effect of escitalopram on an acetic acid-induced ulcerative colitis model. Clin Exp Pharmacol Physiol 48:782–790. https://doi.org/10.1111/1440-1681.13474
Farahbakhsh Z, Radahmadi M (2022) The protective effects of escitalopram on synaptic plasticity in the CA1 region of chronically stressed and non-stressed male rats. Int J Dev Neurosci 82:747–757. https://doi.org/10.1002/jdn.10224
Grolli RE, Bertollo AG, Behenck JP et al (2023) Quetiapine effect on depressive-like behaviors, oxidative balance, and inflammation in serum of rats submitted to chronic stress. Naunyn Schmiedebergs Arch Pharmacol 396:1423–1433. https://doi.org/10.1007/s00210-023-02406-8
Lorenzi H, Matos FJA (2008) Plantas Medicinais no Brasil. In: Nativas e Exóticas, 2nd edn. Instituto Plantarum, Nova Odessa, SP
Alonso J (2004) Tratado de fitofármacos y nutracéuticos, 1st edn. Corpus, Rosario
Bobade V, Bodhankar SL, Aswar U et al (2015) Prophylactic effects of asiaticoside-based standardized extract of Centella asiatica (L.) Urban leaves on experimental migraine: Involvement of 5HT1A/1B receptors. Chin J Nat Med 13:274–282. https://doi.org/10.1016/S1875-5364(15)30014-5
Boondam Y, Songvut P, Tantisira MH et al (2019) Inverted U-shaped response of a standardized extract of Centella asiatica (ECa 233) on memory enhancement. Sci Rep 9:8404. https://doi.org/10.1038/s41598-019-44867-z
Johnson W, Bergfeld WF, Belsito DV et al (2023) Safety assessment of Centella asiatica -derived ingredients as used in cosmetics. Int J Toxicol 42:5S-22S. https://doi.org/10.1177/10915818231158272
James J, Dubery I (2009) Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 14:3922–3941. https://doi.org/10.3390/molecules14103922
Bylka W, Znajdek-Awiżeń P, Studzińska-Sroka E, Brzezińska M (2013) Centella asiatica in cosmetology. Adv Dermatol Allergol 1:46–49. https://doi.org/10.5114/pdia.2013.33378
Devi G, Balasundaram C, Harikrishnan R (2020) Effect of madecassic acid on innate-adaptive immune response and cytokine gene expression in Labeo rohita against Argulus siamensis. MedDocs EBooks
Nasir M, Habsah M, Adzim M et al (2015) Acute effects of triterpene compounds on locomotor performance and Morris water maze tasks in Spraque-Dawley rats. Biomed Res 16:304–310
Réus GZ, Abelaira HM, dos Santos MAB et al (2013) Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res 256:451–456. https://doi.org/10.1016/j.bbr.2013.08.041
Porsolt RD (2000) Animal models of depression: utility for transgenic research. Rev Neurosci 11. https://doi.org/10.1515/REVNEURO.2000.11.1.53
Al-Mousawi AM, Kulp GA, Branski LK et al (2010) Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock 34:261–268. https://doi.org/10.1097/SHK.0b013e3181d8e2a6
Marquardt N, Feja M, Hünigen H et al (2018) Euthanasia of laboratory mice: are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS ONE 13:e0203793. https://doi.org/10.1371/journal.pone.0203793
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 7th edn. Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam, Boston
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356. https://doi.org/10.1016/0003-2697(77)90043-4
Suzuki K, Ota H, Sasagawa S et al (1983) Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132:345–352. https://doi.org/10.1016/0003-2697(83)90019-2
Jentzsch AM, Bachmann H, Fürst P, Biesalski HK (1996) Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 20:251–256. https://doi.org/10.1016/0891-5849(95)02043-8
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
De Souza LM, Cipriani TR, Iacomini M et al (2008) HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J Pharm Biomed Anal 47:59–67
Zanatta MEDC, Miorando D, Stefller AM, et al (2021) Gastroprotective effects of the aqueous extract from taraxacum officinale in rats using ultrasound, histology, and biochemical analysis. Evid Based Complement Alternat Med 2021:1–13. https://doi.org/10.1155/2021/8987232
Engels C, Gräter D, Esquivel P et al (2012) Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res Int 46:557–562. https://doi.org/10.1016/j.foodres.2011.04.003
Falcão SI, Vale N, Gomes P et al (2013) phenolic profiling of portuguese propolis by LC–MS spectrometry:Uncommon propolis rich in flavonoid glycosides. Phytochem Anal 24:309–318. https://doi.org/10.1002/pca.2412
Vallverdú-Queralt A, Jáuregui O, Di Lecce G et al (2011) Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC–ESI-QTOF). Food Chem 129:877–883. https://doi.org/10.1016/j.foodchem.2011.05.038
Attia YM, El-Kersh DM, Wagdy HA, Elmazar MM (2018) Verbascoside: identification, quantification, and potential sensitization of colorectal cancer cells to 5-FU by targeting PI3K/AKT pathway. Sci Rep 8:16939. https://doi.org/10.1038/s41598-018-35083-2
Wijeweera G, Wijekoon N, Gonawala L et al (2023) Therapeutic implications of some natural products for neuroimmune diseases: a narrative of clinical studies review. Evid Based Complement Alternat Med 2023:1–18. https://doi.org/10.1155/2023/5583996
Puttarak P, Dilokthornsakul P, Saokaew S et al (2017) Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: a systematic review and meta-analysis. Sci Rep 7:10646. https://doi.org/10.1038/s41598-017-09823-9
Songvut P, Chariyavilaskul P, Tantisira M, Khemawoot P (2019) Safety and pharmacokinetics of standardized extract of Centella asiatica (ECa 233) capsules in healthy Thai volunteers: a phase 1 clinical study. Planta Med 85:483–490. https://doi.org/10.1055/a-0835-6671
Réus GZ, Silva RH, de Moura AB et al (2019) Early maternal deprivation induces microglial activation, alters glial fibrillary acidic protein immunoreactivity and indoleamine 2,3-dioxygenase during the development of offspring rats. Mol Neurobiol 56:1096–1108. https://doi.org/10.1007/s12035-018-1161-2
Arauchi R, Hashioka S, Tsuchie K et al (2018) Gunn rats with glial activation in the hippocampus show prolonged immobility time in the forced swimming test and tail suspension test. Brain Behav 8:e01028. https://doi.org/10.1002/brb3.1028
Detke MJ, Lucki I (1995) Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73:43–46. https://doi.org/10.1016/0166-4328(96)00067-8
Haraguchi A, Fukuzawa M, Iwami S et al (2018) Night eating model shows time-specific depression-like behavior in the forced swimming test. Sci Rep 8:1081. https://doi.org/10.1038/s41598-018-19433-8
Ignácio ZM, Réus GZ, Abelaira HM et al (2017) Quetiapine treatment reverses depressive-like behavior and reduces DNA methyltransferase activity induced by maternal deprivation. Behav Brain Res 320:225–232. https://doi.org/10.1016/j.bbr.2016.11.044
Khalifeh S, Khodagholi F, Moghtadaei M et al (2019) Effects of maternal deprivation on anxiety, depression, and empathy in male and female offspring of Wistar rats in the face of novel objects. Galen Med J 8:1093. https://doi.org/10.31661/gmj.v0i0.1093
Réus GZ, Fernandes GC, de Moura AB et al (2017) Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J Psychiatr Res 95:196–207. https://doi.org/10.1016/j.jpsychires.2017.08.020
Ignácio ZM, Réus GZ, Quevedo J et al (2017) Maternal deprivation. In: Reference module in neuroscience and biobehavioral psychology, vol 1. Elsevier, pp. 1–12
Sun S, Lin D, Goldberg S et al (2022) A mindfulness-based mobile health (mHealth) intervention among psychologically distressed university students in quarantine during the COVID-19 pandemic: a randomized controlled trial. J Couns Psychol 69:157–171. https://doi.org/10.1037/cou0000568
Matchkov VV, Kravtsova VV, Wiborg O et al (2015) Chronic selective serotonin reuptake inhibition modulates endothelial dysfunction and oxidative state in rat chronic mild stress model of depression. Am J Physiol-Regul Integr Comp Physiol 309:R814–R823. https://doi.org/10.1152/ajpregu.00337.2014
Munzer A, Sack U, Mergl R et al (2013) Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins 5:2227–2240. https://doi.org/10.3390/toxins5112227
Seo MK, Choi CM, McIntyre RS et al (2017) Effects of escitalopram and paroxetine on mTORC1 signaling in the rat hippocampus under chronic restraint stress. BMC Neurosci 18:39. https://doi.org/10.1186/s12868-017-0357-0
Wolkowitz OM, Wolf J, Shelly W et al (2011) Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1623–1630. https://doi.org/10.1016/j.pnpbp.2011.06.013
Lee B, Sur B, Kwon S et al (2013) Chronic administration of catechin decreases depression and anxiety-like behaviors in a rat model using chronic corticosterone injections. Biomol Ther 21:313–322. https://doi.org/10.4062/biomolther.2013.004
Gould TD, Dao DT, Kovacsics CE (2009) The open field test. In: Gould TD (ed) Mood and Anxiety Related Phenotypes in Mice. Humana Press, Totowa, NJ, pp 1–20
Pumpaisalchai W, Kaewichit S, Taesothikul T et al (2005) The antidepressive effect of Barakol in the forced-swimming test. 4:191–198
Detke MJ, Johnson J, Lucki I (1997) Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 5:107–112. https://doi.org/10.1037/1064-1297.5.2.107
Krügel U, Fischer J, Radicke S et al (2013) Antidepressant effects of TNF-α blockade in an animal model of depression. J Psychiatr Res 47:611–616. https://doi.org/10.1016/j.jpsychires.2013.01.007
Tsoukalas D, Zlatian O, Mitroi M et al (2021) A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J Clin Med 10:624. https://doi.org/10.3390/jcm10040624
Schroder JD, de Araújo JB, de Oliveira T et al (2022) Telomeres: the role of shortening and senescence in major depressive disorder and its therapeutic implications. Rev Neurosci 33:227–255. https://doi.org/10.1515/revneuro-2021-0070
Szebeni A, Szebeni K, DiPeri T et al (2014) Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol 17:1579–1589. https://doi.org/10.1017/S1461145714000698
Kordinas V, Ioannidis A, Chatzipanagiotou S (2016) The telomere/telomerase system in chronic inflammatory diseases. Cause or Effect? Genes 7:E60. https://doi.org/10.3390/genes7090060
Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. https://doi.org/10.1097/PSY.0b013e3181907c1b
Maes M, Bosmans E, De Jongh R et al (1997) Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9:853–858. https://doi.org/10.1006/cyto.1997.0238
Snyder JS, Soumier A, Brewer M et al (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. https://doi.org/10.1038/nature10287
Ignácio ZM, da Silva RS, Plissari ME et al (2019) Physical exercise and neuroinflammation in major depressive disorder. Mol Neurobiol 56:8323–8335. https://doi.org/10.1007/s12035-019-01670-1
Won J-H, Shin J-S, Park H-J et al (2010) Anti-inflammatory effects of madecassic acid via the suppression of NF- κ B pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med 76:251–257. https://doi.org/10.1055/s-0029-1186142
Hsu Y-M, Hung Y, Hu L et al (2015) Anti-diabetic effects of madecassic acid and rotundic acid. Nutrients 7:10065–10075. https://doi.org/10.3390/nu7125512
Masola B, Oguntibeju OO, Oyenihi AB (2018) Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed Pharmacother 101:447–457. https://doi.org/10.1016/j.biopha.2018.02.115
Wang N, Li R, Feng B et al (2022) Chicoric acid prevents neuroinflammation and neurodegeneration in a mouse Parkinson’s disease model: immune response and transcriptome profile of the spleen and colon. Int J Mol Sci 23:2031. https://doi.org/10.3390/ijms23042031
Feng T, McEvoy JP, Miller BJ (2020) Longitudinal study of inflammatory markers and psychopathology in schizophrenia. Schizophr Res 224:58–66. https://doi.org/10.1016/j.schres.2020.10.003
Muriach M, Flores-Bellver M, Romero FJ, Barcia JM (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev 2014:e102158. https://doi.org/10.1155/2014/102158
da Silva LA, Tortelli L, Motta J et al (2019) Effects of aquatic exercise on mental health, functional autonomy and oxidative stress in depressed elderly individuals: a randomized clinical trial. Clinics 74:e322. https://doi.org/10.6061/clinics/2019/e322
Syed Umesalma SA (2015) Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain- histopathological, and biochemical approach. J Mol Biomark Diagn 06. https://doi.org/10.4172/2155-9929.1000212
Arora R, Kumar R, Agarwal A et al (2018) Comparison of three different extracts of Centella asiatica for anti-amnesic, antioxidant and anticholinergic activities: in vitro and in vivo study. Biomed Pharmacother 105:1344–1352. https://doi.org/10.1016/j.biopha.2018.05.156
De Groote D, Van Belleghem K, Devière J et al (2012) Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab 61:15–24. https://doi.org/10.1159/000338634
Kazemi M, Lalooha F, Nooshabadi MR et al (2021) Randomized double blind clinical trial evaluating the ellagic acid effects on insulin resistance, oxidative stress and sex hormones levels in women with polycystic ovarian syndrome. J Ovarian Res 14:100. https://doi.org/10.1186/s13048-021-00849-2
Sen CK, Packer L (2000) Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 72:653S-669S. https://doi.org/10.1093/ajcn/72.2.653S
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:1–26. https://doi.org/10.1155/2012/736837
Dionisie V, Ciobanu AM, Toma VA et al (2021) Escitalopram targets oxidative stress, caspase-3, BDNF and MeCP2 in the hippocampus and frontal cortex of a rat model of depression induced by chronic unpredictable mild stress. Int J Mol Sci 22:7483. https://doi.org/10.3390/ijms22147483
Cimen B, Gumus CB, Cetin I et al (2015) The effects of escitalopram treatment on oxidative/antioxidative parameters in patients with depression. Klin Psikofarmakol Bül-Bull Clin Psychopharmacol 25:272–279. https://doi.org/10.5455/bcp.20150215102247
Acknowledgements
Zuleide Maria Ignácio and Margarete Dulce Bagatini are supported by research grants from the National Council for Scientific and Technological Development (CNPq), Santa Catarina State Research and Innovation Support Foundation—FAPESC, and Federal University of Southern Frontier—UFFS.
Funding
This research was supported by grants from CNPq (ZMI and MDB), FAPESC (ZMI and MDB), and UFFS (ZMI and MDB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Amanda Gollo Bertollo, Maiqueli Eduarda Dama Mingoti, Jesiel de Medeiros, Gilnei Bruno da Silva, Giovana Tamara Capoani, Heloisa Lindemann, Joana Cassol, Daiane Manica, Tacio de Oliveira, Michelle Lima Garcez, Margarete Dulce Bagatini, Lilian Caroline Bohnen, Walter Antônio Roman Junior, and Zuleide Maria Ignácio. Amanda Gollo Bertollo wrote the first draft of the manuscript. All authors commented on the previous version of the manuscript. Zuleide Maria Ignácio reviewed and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Yes, under protocol code 002/CEUA/2021.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bertollo, A.G., Mingoti, M.E.D., de Medeiros, J. et al. Hydroalcoholic Extract of Centella asiatica and Madecassic Acid Reverse Depressive-Like Behaviors, Inflammation and Oxidative Stress in Adult Rats Submitted to Stress in Early Life. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04198-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04198-1