Abstract
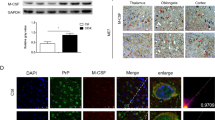
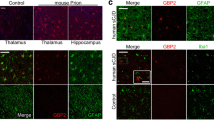
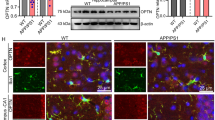
Neuroinflammation is a common pathological feature in a number of neurodegenerative diseases, which is mediated primarily by the activated glial cells. Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NLRP3) inflammasome-associated neuroinflammatory response is mostly considered. To investigate the situation of the NLRP3-related inflammation in prion disease, we assessed the levels of the main components of NLRP3 inflammasome and its downstream biomarkers in the scrapie-infected rodent brain tissues. The results showed that the transcriptional and expressional levels of NLRP3, caspase-1, and apoptosis-associated speck-like protein (ASC) in the brains of scrapie-infected rodents were significantly increased at terminal stage. The increased NLPR3 overlapped morphologically well with the proliferated GFAP-positive astrocytes, but little with microglia and neurons. Using the brain samples collected at the different time-points after infection, we found the NLRP3 signals increased in a time-dependent manner, which were coincidental with the increase of GFAP. Two main downstream cytokines, IL-1β and IL-18, were also upregulated in the brains of prion-infected mice. Moreover, the gasdermin D (GSDMD) levels, particularly the levels of GSDMD-NT, in the prion-infected brain tissues were remarkably increased, indicating activation of cell pyroptosis. The GSDMD not only co-localized well with the astrocytes but also with neurons at terminal stage, also showing a time-dependent increase after infection. Those data indicate that NLRP3 inflammasomes were remarkably activated in the infected brains, which is largely mediated by the proliferated astrocytes. Both astrocytes and neurons probably undergo a pyroptosis process, which may help the astrocytes to release inflammatory factors and contribute to neuron death during prion infection.






Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Ma Y, Shi Q, Xiao K, Wang J, Chen C, Gao LP, Gao C, Dong XP (2019) Stimulations of the culture medium of activated microglia and TNF-alpha on a scrapie-infected cell line decrease the cell viability and induce marked necroptosis that also occurs in the brains from the patients of human prion diseases. ACS Chem Neurosci 10(3):1273–1283. https://doi.org/10.1021/acschemneuro.8b00354
Zhou DH, Wang J, Xiao K, Wu YZ, Maimaitiming A, Hu C, Gao LP, Chen J, Gao C, Chen C, Shi Q, Dong XP (2020) Stilbene compounds inhibit the replications of various strains of prions in the levels of cell culture, PMCA, and RT-QuIC possibly via molecular binding. ACS Chem Neurosci 11(14):2117–2128. https://doi.org/10.1021/acschemneuro.0c00218
Mead S, Khalili-Shirazi A, Potter C, Mok T, Nihat A, Hyare H, Canning S, Schmidt C, Campbell T, Darwent L, Muirhead N, Ebsworth N, Hextall P, Wakeling M, Linehan J, Libri V, Williams B, Jaunmuktane Z, Brandner S, Rudge P, Collinge J (2022) Prion protein monoclonal antibody (PRN100) therapy for Creutzfeldt-Jakob disease: evaluation of a first-in-human treatment programme. Lancet Neurol 21(4):342–354. https://doi.org/10.1016/s1474-4422(22)00082-5
Ma Y, Shi Q, Wang J, Xiao K, Sun J, Lv Y, Guo M, Zhou W, Chen C, Gao C, Zhang BY, Dong XP (2017) Reduction of NF-κB (p65) in scrapie-infected cultured cells and in the brains of scrapie-infected rodents. ACS Chem Neurosci 8(11):2535–2548. https://doi.org/10.1021/acschemneuro.7b00273
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De Strooper B, Thal DR (2023) Pyroptosis in Alzheimer’’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 145(2):175–195. https://doi.org/10.1007/s00401-022-02528-y
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, Griep A, Santarelli F, Brosseron F, Opitz S, Stunden J, Merten M, Kayed R, Golenbock DT, Blum D, Latz E, Buée L, Heneka MT (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575(7784):669–673. https://doi.org/10.1038/s41586-019-1769-z
Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, Zheng P, Xie P, Zhang Z, Yao H (2019) Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 7(1):116. https://doi.org/10.1186/s40168-019-0733-3
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678. https://doi.org/10.1038/nature11729
Barnett KC, Li S, Liang K, Ting JP (2023) A 360° view of the inflammasome: mMechanisms of activation, cell death, and diseases. Cell 186(11):2288–2312. https://doi.org/10.1016/j.cell.2023.04.025
Nozaki K, Maltez VI, Rayamajhi M, Tubbs AL, Mitchell JE, Lacey CA, Harvest CK, Li L, Nash WT, Larson HN, McGlaughon BD, Moorman NJ, Brown MG, Whitmire JK, Miao EA (2022) Caspase-7 activates ASM to repair gasdermin and perforin pores. Nature 606(7916):960–967. https://doi.org/10.1038/s41586-022-04825-8
Knorr M, Hoppe J, Steuhl KP, Dartsch PC (1992) Effect of PDGF-AB heterodimer on a corneal epithelial cell line. Eur J Cell Biol 57(2):202–209
Ma X, Hao J, Wu J, Li Y, Cai X, Zheng Y (2022) Prussian blue nanozyme as a pyroptosis inhibitor alleviates neurodegeneration. Adv Mater 34(15):e2106723. https://doi.org/10.1002/adma.202106723
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610):153–158. https://doi.org/10.1038/nature18629
Wang X, Li X, Liu S, Brickell AN, Zhang J, Wu Z, Zhou S, Ding Z (2020) PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res Cardiol 115(6):66. https://doi.org/10.1007/s00395-020-00832-w
Siew JJ, Chen HM, Chen HY, Chen HL, Chen CM, Soong BW, Wu YR, Chang CP, Chan YC, Lin CH, Liu FT, Chern Y (2019) Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’’s disease. Nat Commun 10(1):3473. https://doi.org/10.1038/s41467-019-11441-0
Wu AG, Zhou XG, Qiao G, Yu L, Tang Y, Yan L, Qiu WQ, Pan R, Yu CL, Law BY, Qin DL, Wu JM (2021) Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev 65:101202. https://doi.org/10.1016/j.arr.2020.101202
Xu F, Wu Y, Yang Q, Cheng Y, Xu J, Zhang Y, Dai H, Wang B, Ma Q, Chen Y, Lin F, Wang C (2022) Engineered extracellular vesicles with SHP2 high expression promote mitophagy for Alzheimer’’s disease treatment. Adv Mater 34(49):e2207107. https://doi.org/10.1002/adma.202207107
Panicker N, Kam TI, Wang H, Neifert S, Chou SC, Kumar M, Brahmachari S, Jhaldiyal A, Hinkle JT, Akkentli F, Mao X, Xu E, Karuppagounder SS, Hsu ET, Kang SU, Pletnikova O, Troncoso J, Dawson VL, Dawson TM (2022) Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’’s disease. Neuron 110(15):2422-2437.e2429. https://doi.org/10.1016/j.neuron.2022.05.009
Lawrence G, Holley CL, Schroder K (2022) Parkinson’’s disease: connecting mitochondria to inflammasomes. Trends Immunol 43(11):877–885. https://doi.org/10.1016/j.it.2022.09.010
Xiao K, Zhang BY, Zhang XM, Wang J, Chen C, Chen LN, Lv Y, Shi Q, Dong XP (2016) Re-infection of the prion from the scrapie-infected cell line SMB-S15 in three strains of mice, CD1, C57BL/6 and Balb/c. Int J Mol Med 37(3):716–726. https://doi.org/10.3892/ijmm.2016.2465
Kim YS, Carp RI, Callahan SM, Wisniewski HM (1987) Incubation periods and survival times for mice injected stereotaxically with three scrapie strains in different brain regions. J Gen Virol 68(Pt 3):695–702. https://doi.org/10.1099/0022-1317-68-3-695
Shi Q, Zhang BY, Gao C, Zhang J, Jiang HY, Chen C, Han J, Dong XP (2012) Mouse-adapted scrapie strains 139A and ME7 overcome species barrier to induce experimental scrapie in hamsters and changed their pathogenic features. Virol J 9:63. https://doi.org/10.1186/1743-422x-9-63
Chou WC, Jha S, Linhoff MW, Ting JP (2023) The NLR gene family: from discovery to present day. Nat Rev Immunol 23(10):635–654. https://doi.org/10.1038/s41577-023-00849-x
Kibby EM, Conte AN, Burroughs AM, Nagy TA, Vargas JA, Whalen LA, Aravind L, Whiteley AT (2023) Bacterial NLR-related proteins protect against phage. Cell 186(11):2410-2424.e2418. https://doi.org/10.1016/j.cell.2023.04.015
Singh J, Habean ML, Panicker N (2023) Inflammasome assembly in neurodegenerative diseases. Trends Neurosci 46(10):814–831. https://doi.org/10.1016/j.tins.2023.07.009
Shao S, Chen C, Shi G, Zhou Y, Wei Y, Fan N, Yang Y, Wu L, Zhang T (2021) Therapeutic potential of the target on NLRP3 inflammasome in multiple sclerosis. Pharmacol Ther 227:107880. https://doi.org/10.1016/j.pharmthera.2021.107880
Osso LA, Chan JR (2015) Astrocytes underlie neuroinflammatory memory impairment. Cell 163(7):1574–1576. https://doi.org/10.1016/j.cell.2015.12.001
Zhu J, Hu Z, Han X, Wang D, Jiang Q, Ding J, Xiao M, Wang C, Lu M, Hu G (2018) Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of β-arrestin2 and NLRP3. Cell Death Differ 25(11):2037–2049. https://doi.org/10.1038/s41418-018-0127-2
Giordano AMS, Luciani M, Gatto F, Abou Alezz M, Beghè C, Della Volpe L, Migliara A, Valsoni S, Genua M, Dzieciatkowska M, Frati G, Tahraoui-Bories J, Giliani SC, Orcesi S, Fazzi E, Ostuni R, D’Alessandro A, Di Micco R, Merelli I, Lombardo A, Reijns MAM, Gromak N, Gritti A, Kajaste-Rudnitski A (2022) DNA damage contributes to neurotoxic inflammation in Aicardi-Goutières syndrome astrocytes. J Exp Med 219(4):e20211121. https://doi.org/10.1084/jem.20211121
Shi F, Yang L, Kouadir M, Yang Y, Wang J, Zhou X, Yin X, Zhao D (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 9:73. https://doi.org/10.1186/1742-2094-9-73
Lai M, Yao H, Shah SZA, Wu W, Wang D, Zhao Y, Wang L, Zhou X, Zhao D, Yang L (2018) The NLRP3-caspase 1 inflammasome negatively regulates autophagy via TLR4-TRIF in prion peptide-infected microglia. Front Aging Neurosci 10:116. https://doi.org/10.3389/fnagi.2018.00116
Li S, Fang Y, Zhang Y, Song M, Zhang X, Ding X, Yao H, Chen M, Sun Y, Ding J, Wang Q, Lu M, Wu G, Hu G (2022) Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep 41(4):111532. https://doi.org/10.1016/j.celrep.2022.111532
Niu T, De Rosny C, Chautard S, Rey A, Patoli D, Groslambert M, Cosson C, Lagrange B, Zhang Z, Visvikis O, Hacot S, Hologne M, Walker O, Wong J, Wang P, Ricci R, Henry T, Boyer L, Petrilli V, Py BF (2021) NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat Commun 12(1):5862. https://doi.org/10.1038/s41467-021-26142-w
Hochheiser IV, Pilsl M, Hagelueken G, Moecking J, Marleaux M, Brinkschulte R, Latz E, Engel C, Geyer M (2022) Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 604(7904):184–189. https://doi.org/10.1038/s41586-022-04467-w
Wang C, Yang T, Xiao J, Xu C, Alippe Y, Sun K, Kanneganti TD, Monahan JB, Abu-Amer Y, Lieberman J, Mbalaviele G (2021) NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci Immunol 6(64):eabj3859. https://doi.org/10.1126/sciimmunol.abj3859
Wang J, Zhang BY, Zhang J, Xiao K, Chen LN, Wang H, Sun J, Shi Q, Dong XP (2016) Treatment of SMB-S15 cells with resveratrol efficiently removes the PrP(Sc) accumulation in vitro and prion infectivity in vivo. Mol Neurobiol 53(8):5367–5376. https://doi.org/10.1007/s12035-015-9464-z
Sigurdson CJ, Bartz JC, Glatzel M (2019) Cellular and molecular mechanisms of prion disease. Annu Rev Pathol 14:497–516. https://doi.org/10.1146/annurev-pathmechdis-012418-013109
Xu Y, Tian C, Wang SB, Xie WL, Guo Y, Zhang J, Shi Q, Chen C, Dong XP (2012) Activation of the macroautophagic system in scrapie-infected experimental animals and human genetic prion diseases. Autophagy 8(11):1604–1620. https://doi.org/10.4161/auto.21482
Trudler D, Nazor KL, Eisele YS, Grabauskas T, Dolatabadi N, Parker J, Sultan A, Zhong Z, Goodwin MS, Levites Y, Golde TE, Kelly JW, Sierks MR, Schork NJ, Karin M, Ambasudhan R, Lipton SA (2021) Soluble α-synuclein-antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc Natl Acad Sci U S A 118(15):e2025847118. https://doi.org/10.1073/pnas.2025847118
Yang T, Zhang L, Shang Y, Zhu Z, Jin S, Guo Z, Wang X (2022) Concurrent suppression of Aβ aggregation and NLRP3 inflammasome activation for treating Alzheimer’’s disease. Chem Sci 13(10):2971–2980. https://doi.org/10.1039/d1sc06071f
Su L, Zhang J, Gomez H, Kellum JA, Peng Z (2023) Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 19(2):401–414. https://doi.org/10.1080/15548627.2022.2084862
Zhu M, Du L, Zhao R, Wang HY, Zhao Y, Nie G, Wang RF (2020) Cell-penetrating nanoparticles activate the inflammasome to enhance antibody production by targeting microtubule-associated protein 1-light chain 3 for degradation. ACS Nano 14(3):3703–3717. https://doi.org/10.1021/acsnano.0c00962
Harrison D, Billinton A, Bock MG, Doedens JR, Gabel CA, Holloway MK, Porter RA, Reader V, Scanlon J, Schooley K, Watt AP (2023) Discovery of clinical candidate NT-0796, a brain-penetrant and highly potent NLRP3 inflammasome inhibitor for neuroinflammatory disorders. J Med Chem. https://doi.org/10.1021/acs.jmedchem.3c01398
. Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases Nat Med 21 (3):248-255 https://doi.org/10.1038/nm.3806
Funding
This work was supported by the grant (2021SKLID101, 2019SKLID501, 2019SKLID603) from the State Key Laboratory for Infectious Disease Prevention and Control, China CDC.
Author information
Authors and Affiliations
Contributions
Dong-Hua Zhou, Qi Shi, and Xiao-Ping Dong wrote the main manuscript text. Xiao-Xi Jia, Yue-Zhang Wu, Wei-Wei Zhang, Yuan Wang, Dong-Lin Liang, Li-Ping Gao, King Xiao, and Can Chen participated in analyzing the results. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
Approved. Details are provided in the beginning of the “Materials and Methods” section.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, DH., Jia, XX., Wu, YZ. et al. Aberrant Enhanced NLRP3 Inflammasomes and Cell Pyroptosis in the Brains of Prion-Infected Rodent Models Are Largely Associated with the Proliferative Astrocytes. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04169-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04169-6