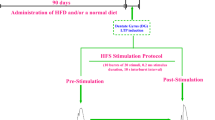
Abstract
Maternal nutrition was recognized as a significant part of brain growth and maturation in most mammalian species. Timely intervention with suitable nutraceuticals would provide long-term health benefits. We aim to unravel the molecular mechanisms of perinatal undernutrition-induced impairments in cognition and synaptic plasticity, employing animal model based on dietary nutraceutical supplementation. We treated undernourished dams at their gestational, lactational, and at both the time point with Astaxanthin (AsX) and Docosahexaenoic acid (DHA), and their pups were used as experimental animals. We evaluated the cognitive function by subjecting the pups to behavioral tests in their adult life. In addition, we assessed the expression of genes in the hippocampus related to cognitive function and synaptic plasticity. Our results showed downregulation of Brain-derived neurotrophic factor (BDNF), Neurotrophin-3 (NT-3), cAMP response-element-binding protein (CREB), and uncoupling protein-2 (UCP2) gene expression in pups born to undernourished dams in their adult life, which AsX and DHA modulated. Maternal AsX and DHA supplementation ameliorated the undernutrition-induced learning impairment in novel object recognition (NOR) tests and partially baited radial arm maze (RAM) tasks in offspring’s. The expressions of Synapsin-1 and PSD-95 decreased in perinatally undernourished groups compared to control and AsX-DHA treated groups at CA1, CA2, CA3, and DG. AsX and DHA supplementation upregulated BDNF, NT-3, CREB, and UCP2 gene expressions in perinatally undernourished rats, which are involved in intracellular signaling cascades like Ras, PI3K, and PLC. The results of our study give new insights into neuronal differentiation, survival, and plasticity, indicating that the perinatal period is the critical time for reversing maternal undernutrition-induced cognitive impairment in offspring’s.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are included in the manuscript and supplementary file.
Abbreviations
- ABSR:
-
Adaptive Bbio-energetic Stress Response
- AsX:
-
Astaxanthin
- BDNF:
-
Brain Derived Neurotrophic Factor
- BSI:
-
Brain Somatic Index
- C:
-
Control
- DC:
-
Drug Control
- CREB:
-
Cyclic AMP Response Element-Binding Protein
- DHA:
-
Docosahexaenoic Acid
- DI:
-
Discrimination Index
- NOR:
-
Novel Object Recognition
- NT-3:
-
Neurotrophin-3
- n-3PUFAs:
-
N-3 long-chain Polyunsaturated Fatty Acids
- PD:
-
Postnatal Day
- PeriUN-AD:
-
Perinatally Undernourished Rats Supplemented with Astaxanthin and Docosahexaenoic Acid
- %CC:
-
Percentage Correct Choice
- Peri UN:
-
Perinatally Undernourished Rats
- PostUN-AD:
-
Postnatally Undernourished Rats Supplemented with Astaxanthin and Docosahexaenoic Acid
- PostUN:
-
Postnatally Undernourished Rats
- PreUN-AD:
-
Prenatally Undernourished Rats Supplemented with Astaxanthin and Docosahexaenoic Acid
- PreUN:
-
Prenatally Undernourished Rats
- PSD-95:
-
Postsynaptic Density Protein 95
- PUFAs:
-
Polyunsaturated Fatty Acids
- RAM:
-
Radial Arm Maze
- RMEs:
-
Reference Memory Errors
- ROS:
-
Reactive Oxygen Species
- TAC:
-
Total Antioxidant Capacity
- UCP2:
-
Uncoupling Protein-2
- VC:
-
Vehicle Control
- VCUN:
-
Perinatally Undernourished Rats Supplemented with Vehicle (Olive Oil)
- WMEs:
-
Working Memory Errors
References
Barbeito-Andrés J, Gleiser PM, Bernal V, Hallgrímsson B, Gonzalez PN (2018) Brain structural networks in mouse exposed to chronic maternal undernutrition. Neuroscience 380:14–26
Lesage J, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Breton C, Deloof S, Vieau D (2006) Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress 9:183–198
Lapiz MDS, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA (2003) Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol 33:13–29
Damodara Gowda KM, Suchetha Kumari N, Ullal H (2018) Nutritional Neuroscience An International Journal on Nutrition, Diet and Nervous System Role of astaxanthin in the modulation of brain-derived neurotrophic factor and spatial learning behavior in perinatally undernourished Wistar rats Role of astaxanthin in the modulation of brain-derived neurotrophic factor and spatial learning behavior in perinatally undernourished Wistar rats. https://doi.org/10.1080/1028415X.2018.1515301
Morgane PJ, Mokler DJ, Galler JR (2002) Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev 26:471–483
Ambati RR, Moi PS, Ravi S, Aswathanarayana RG (2014) Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications - A review. Mar Drugs 12:128–152
Naguib YMA (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 48:1150–1154
Liu X, Osawa T (2007) Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem Biophys Res Commun 357:187–193
Ying C-J, Zhang F, Zhou X-Y, Hu X-T, Chen J, Wen X-R, Sun Y, Zheng K-Y et al (2015) Anti-inflammatory effect of astaxanthin on the sickness behavior induced by diabetes mellitus. Cell Mol Neurobiol 35:1027–1037
Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, Watanabe H (2005) Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull 28:47–52
Lu Y, Xie T, He XX, Mao ZF, Jia LJ, Wang WP, Zhen JL, Liu LM (2015) Astaxanthin rescues neuron loss and attenuates oxidative stress induced by amygdala kindling in adult rat hippocampus. Neurosci Lett 597:49–53
Green P, Glozman S, Kamensky B, Yavin E (1999) Developmental changes in rat brain membrane lipids and fatty acids: The preferential prenatal accumulation of docosahexaenoic acid. J Lipid Res 40:960–966
Ikemoto A, Kobayashi T, Emoto K, Umeda M, Watanabe S, Okuyama H (1999) Effects of docosahexaenoic and arachidonic acids on the synthesis and distribution of aminophospholipids during neuronal differentiation of PC12 Cells
Calderon F, Kim HY (2004) Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 90:979–988
Kim HY, Akbar M, Lau A, Edsall L (2000) Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n–3): Role of phosphatidylserine in antiapoptotic effect. J Biol Chem 275:35215–35223
Carver JD, Benford VJ, Han B, Cantor AB (2001) The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects
Desai A, Kevala K, Kim HY (2014) Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. https://doi.org/10.1371/journal.pone.0086472
Mills JD, Hadley K, Bailes JE (2011) Dietary supplementation with the Omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 68:474–481
Jarrard LE (1993) On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol 60:9–26
Lohof AM, Ip NY, Poo MM, (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363(6427):350–353
Author H, Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. American Association for the Advancement of Science Stable. Science 267:1658–1662
Ramos-Languren LE, Escobar ML (2013) Plasticity and metaplasticity of adult rat hippocampal mossy fibers induced by neurotrophin-3. Eur J Neurosci 37:1248–1259
Bar D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel Howard ER (1995) Aplysia CREW represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change
West AE, Griffith EC, Greenberg ME (2002) Regulation of transcription factors by neuronal activity. Nat Rev Neurosci 3:921–931
Deisseroth K, Mermelstein PG, Xia H, Tsien RW (2003) Signaling from synapse to nucleus: The logic behind the mechanisms. Curr Opin Neurobiol 13:354–365
Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL (2002) Brain mitochondrial uncoupling protein 2 (UCP2): a protectivestress signal in neuronal injury. Biochem Pharmacol 64:363–367
Valadares CT, Fukuda MTH, Françolin-Silva AL, Hernandes AS, Almeida SS (2010) Effects of postnatal protein malnutrition on learning and memory procedures. Nutr Neurosci 13:274–282
Pérez-Garciá G, Guzmán-Quevedo O, Da Silva AR, Bolanõs-Jiménez F (2016) Early malnutrition results in long-lasting impairments in pattern-separation for overlapping novel object and novel location memories and reduced hippocampal neurogenesis. Sci Rep. https://doi.org/10.1038/srep21275
Berardino BG, Ballarini F, Chertoff M, Igaz LM, Cánepa ET (2022) Nutritional stress timing differentially programs cognitive abilities in young adult male mice. Nutr Neurosci 25:286–298
Tau GZ, Peterson BS (2010) Normal development of brain circuits. Neuropsychopharmacology 35:147–168
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Preedy VR, Patel VB (2019) Handbook of famine, starvation, and nutrient deprivation: From biology to policy. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy 1–2407
Rajamoorthi A, LeDuc CA, Thaker VV (2022) The metabolic conditioning of obesity: A review of the pathogenesis of obesity and the epigenetic pathways that “program” obesity from conception. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2022.1032491
Prado EL, Dewey KG (2014) Nutrition and brain development in early life. Nutr Rev 72:267–284
Salminen LE, Paul RH (2014) Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev Neurosci 25:805–819
Catalá A, Díaz M (2016) Editorial: Impact of lipid peroxidation on the physiology and pathophysiology of cell membranes. Front Physiol. https://doi.org/10.3389/fphys.2016.00423
Bhat Agni M, Hegde PS, Ullal H, Damodara Gowda KM (2023) Nutritional efficacy of Astaxanthin in modulating orexin peptides and fatty acid level during adult life of rats exposed to perinatal undernutrition stress. Nutr Neurosci. https://doi.org/10.1080/1028415X.2022.2123184
Carlin G, Chaumontet C, Blachier F et al (2020) Perinatal exposure of rats to a maternal diet with varying protein quantity and quality affects the risk of overweight in female adult offspring. J Nutr Biochem. https://doi.org/10.1016/j.jnutbio.2019.108333
Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2011) Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology 214:477–494
Sandhya T, Sowjanya J, Veeresh B (2012) Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochem Res 37:1121–1131
Shailaja M, Damodara Gowda KM, Vishakh K, SuchethaKumari N (2017) Anti-aging role of curcumin by modulating the inflammatory markers in albino wistar rats. J Natl Med Assoc 109:9–13
Dzhala V, Fowler AJ, DiMarzio BA, Staley KJ, Suh J (2022) Analysis of brain region-specific mRNA synthesis and stability by utilizing adult mouse brain slice culture. STAR Protoc. https://doi.org/10.1016/j.xpro.2022.101349
Cui Y, Cao K, Lin H et al (2020) Early-life stress induces depression-like behavior and synaptic-plasticity changes in a maternal separation rat model: Gender difference and metabolomics study. Front Pharmacol. https://doi.org/10.3389/fphar.2020.00102
Antunes M, Biala G (2012) The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Monk C, Georgieff MK, Osterholm EA (2013) Research review: Maternal prenatal distress and poor nutrition - Mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry 54:115–130
Pandey AK (2017) Disruption of neurosynaptic physiology and neuron network dysfunction in brain disorders: an environmental and occupational health perspective. Act Nerv Super (Praha) 59:61–77
Cho WK, Suh BK (2016) Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr 59:1–7
Georgieff MK, Ramel SE, Cusick SE (2018) Nutritional influences on brain development. Acta Paediatrica Int J Paediatr 107:1310–1321
Liu X, Luo B, Peng W, Xiong F, Yang F, Wu J (2019) Factors affecting the catch-up growth of preterm infants after discharge in China: A multicenter study based on the health belief model. Ital J Pediatr. https://doi.org/10.1186/s13052-019-0674-2
Ranade AV, Hegde PS, Bhat MA, Rai P, Vinodini NA, Aravind A, Prasad TSK, Damodara Gowda KM (2023) Astaxanthin and DHA supplementation ameliorates the proteomic profile of perinatal undernutrition-induced adipose tissue dysfunction in adult life. Sci Rep. https://doi.org/10.1038/s41598-023-38506-x
Sinha S, Patro N, Patro IK (2020) Amelioration of neurobehavioral and cognitive abilities of F1 progeny following dietary supplementation with Spirulina to protein malnourished mothers. Brain Behav Immun 85:69–87
Calikoglu AS, Karayal AF, Joseph D’ercole A (2001) Nutritional regulation of IGF-I expression during brain development in mice. Pediatric Res 49:197–202
Melgar-Locatelli S, de Ceglia M, Mañas-Padilla MC, Rodriguez-Pérez C, Castilla-Ortega E, Castro-Zavala A, Rivera P (2023) Nutrition and adult neurogenesis in the hippocampus: Does what you eat help you remember? Front Neurosci. https://doi.org/10.3389/fnins.2023.1147269
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical Implications. Arch Med Sci 11:1164–1178
Georgieff MK, Brunette KE, Tran PV (2015) Early life nutrition and neural plasticity. Dev Psychopathol 27:411–423
Yan Z, Shi X, Wang H, Si C, Liu Q, Du Y (2021) Neurotrophin-3 promotes the neuronal differentiation of BMSCs and improves cognitive function in a rat model of alzheimer’s disease. Front Cell Neurosci. https://doi.org/10.3389/fncel.2021.629356
Wattez JS, Delahaye F, Barella LF, Dickes-Coopman A, Montel V, Breton C, Mathias P, Foligné B et al (2014) Short-and long-term effects of maternal perinatal undernutrition are lowered by cross-fostering during lactation in the male rat. J Dev Orig Health Dis 5:109–120
Polverino A, Sorrentino P, Pesoli M, Mandolesi L (2021) Nutrition and cognition across the lifetime: an overview on epigenetic mechanisms. AIMS Neurosci 8:448–476
Sakamoto K, Karelina K, Obrietan K (2011) CREB: A multifaceted regulator of neuronal plasticity and protection. J Neurochem 116:1–9
Wang H, Xu J, Lazarovici P, Quirion R, Zheng W (2018) cAMP response element-binding protein (CREB): A possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2018.00255
Kadosh KC, Muhardi L, Parikh P, Basso M, Mohamed HJJ, Prawitasari T, Samuel F, Ma G et al (2021) Nutritional support of neurodevelopment and cognitive function in infants and young children—an update and novel insights. Nutrients 13:1–26
Kandel ER (2012) The molecular biology of memory: CAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. https://doi.org/10.1186/1756-6606-5-14
Loui A, Raab A, Maier RF, Brätter P, Obladen M (2010) Trace elements and antioxidant enzymes in extremely low birthweight infants. J Trace Elem Med Biol 24:111–118
Likhar A, Patil MS (2022) Importance of maternal nutrition in the first 1,000 days of life and its effects on child development: A narrative review. Cureus. https://doi.org/10.7759/cureus.30083
Sreedhar A, Zhao Y (2017) Uncoupling protein 2 and metabolic diseases. Mitochondrion 34:135–140
Pierelli G, Stanzione R, Forte M, Migliarino S, Perelli M, Volpe M, Rubattu S (2017) Uncoupling protein 2: A key player and a potential therapeutic target in vascular diseases. Oxid Med Cell Longev. https://doi.org/10.1155/2017/7348372
Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L (2018) Role of ROS and nutritional antioxidants in human diseases. Front Physiol. https://doi.org/10.3389/fphys.2018.00477
Mehta SL, Li PA (2009) Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb Blood Flow Metab 29:1069–1078
Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60:748–766
Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P (1999) Synapsins as regulators of neurotransmitter release. In: Philosophical Transactions of the Royal Society B: Biological Sciences. Royal Society, 269–279
Evstratova A, Tóth K (2014) Information processing and synaptic plasticity at hippocampal mossy fiber terminals. Front Cell Neurosci. https://doi.org/10.3389/fncel.2014.00028
Mirza FJ, Zahid S (2018) The role of synapsins in neurological disorders. Neurosci Bull 34:349–358
Mardones MD, Jorquera PV, Herrera-Soto A, Ampuero E, Bustos FJ, van Zundert B, Varela-Nallar L (2019) PSD95 regulates morphological development of adult-born granule neurons in the mouse hippocampus. J Chem Neuroanat 98:117–123
Acknowledgements
The authors acknowledge the support of the institutional animal care facility, Nitte (Deemed to be University) and laboratory facilities at Nitte University Centre for Science Education and Research, and Biotechnology Unit, Dept of Biosciences, Mangalore University. Auhors acknowledge the financial support from Indian Council of Medical Research (No. 5/9/1220/2019-Nut.).
Authors also acknowledge the constructive recommendations of the honorable reviwers.
Funding
This study was supported by grants from the Indian Council of Medical Research (No. 5/9/1220/2019-Nut.). Corresponding Author, Damodara Gowda K M has received research support from Indian Council of Medical Research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. M.B.A. and P.S. H. carried out animal handling and treatment experiments, performed statistical analysis and preparation of figures and tables, and contributed to the drafting and editing of the manuscript. D.G. K M., P. R., and M.S. provided scientific advice and contributed to designing the research work, conceptualizing the ideas, preparing tables and figures, reviewing the analyzed data, and drafting and editing the manuscript. M.S. supervised behavioral experiments. D.G.K. M. led the entire research. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal experiments were approved by the Institutional Animal Ethics Committee, KS Hegde Medical Academy, Nitte (Deemed to be University), Mangalore (approval number Ref. KSHEMA/IAEC/05/2019).
Consent for Publication
Not applicable as there was no content obtained from other sources. All the authors consented for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agni, M.B., Hegde, P.S., Rai, P. et al. Astaxanthin and DHA Supplementation Modulates the Maternal Undernutrition-induced Impairment of Cognitive Behavior and Synaptic Plasticity in Adult Life of Offspring’s -Exploring the Molecular Mechanism. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04147-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04147-y