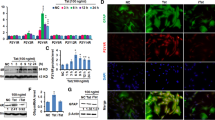
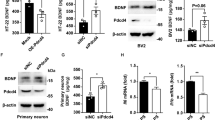
Abstract
Influenza A virus (IAV) infection, which leads to millions of new cases annually, affects many tissues and organs of the human body, including the central nervous system (CNS). The incidence of affective disorders has increased after the flu pandemic; however, the potential mechanism has not been elucidated. PB1-F2, a key virulence molecule of various influenza virus strains, has been shown to inhibit cell proliferation and induce host inflammation; however, its role in the CNS has not been studied. In this study, we constructed and injected PB1-F2 into the hippocampal dentate gyrus (DG), a region closely associated with newborn neurons and neural development, to evaluate its influence on negative affective behaviors and learning performance in mice. We observed anxiety- and depression-like behaviors, but not learning impairment, in mice injected with PB1-F2. Furthermore, pull-down and mass spectrometry analyses identified several potential PB1-F2 binding proteins, and enrichment analysis suggested that the most affected function was neural development. Morphological and western blot studies revealed that PB1-F2 inhibited cell proliferation and oligodendrocyte development, impaired myelin formation, and interfered with synaptic plasticity in DG. Taken together, our results demonstrated that PB1-F2 induces affective disorders by inhibiting oligodendrocyte development and regulating synaptic plasticity in the DG after IAV infection, which lays the foundation for developing future cures of affective disorders after IAV infection.






Similar content being viewed by others
Data Availability
The authors will provide the data under a reasonable request.
Abbreviations
- IAV:
-
Influenza A virus
- PB1-F2:
-
protein 1-frame 2
- CNS:
-
central nervous system
- DG:
-
dentate gyrus
- IL-1β:
-
interleukin-1β
- IL-6:
-
interleukin-6
- TNF-α:
-
tumor necrosis factor-α
- LTP:
-
long-term potentiation
- ANT3:
-
adenine nucleotide translocator 3
- VDAC1:
-
voltage-dependent anion channel 1
- NLRX1:
-
nucleotide-binding oligomerization domain-like receptor X1
- TUFM:
-
Tu translation elongation factor, mitochondrial
- LC3B:
-
microtubule associated protein 1 light chain 3 beta
- NLRP3:
-
NLR family pyrin domain containing 3
- PDGFRα:
-
platelet derived growth factor receptor alpha
- OLIG2:
-
oligodendrocyte lineage transcription factor 2
- GAP43:
-
growth associated protein-43
- SOX10:
-
SRY-box containing gene10
- MAP2:
-
microtubule associated protein-2
- NMDA:
-
N-methyl-D-aspartate
- AMPA:
-
alberta magazine publishers association
- BDNF:
-
brain derived nerve factor
- CaMKII:
-
calmodulin dependent protein kinase II
- PSD95:
-
postsynaptic density-95
- GluN2A:
-
glutamate receptor ionotropic, NMDA 2A
- GluN2B :
-
glutamate receptor ionotropic, NMDA 2B
- GluA1:
-
glutamate receptor ionotropic, AMPA 1
- GluA2:
-
glutamate receptor ionotropic, AMPA 2
- MCT1:
-
monocarboxylate transporter 1
- OPCs:
-
oligodendrocyte progenitor cells
- OLs:
-
mature oligodendrocytes
References
Fan H, Walker AP, Carrique L et al (2019) Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature 573(7773):287–290. https://doi.org/10.1038/s41586-019-1530-7
Wang XH, Zeng ZY, Zhang ZY et al (2018) The appropriate combination of hemagglutinin and neuraminidase prompts the predominant H5N6 highly pathogenic avian influenza virus in birds. Front Microbiol 9:1088. https://doi.org/10.3389/fmicb.2018.01088
Courtney DG, Kennedy EM, Dumm RE et al (2017) Epitranscriptomic enhancement of influenza A virus gene expression and replication. Cell Host Microbe 22(3):377–386.e5. https://doi.org/10.1016/j.chom.2017.08.004
Matrosovich MN, Matrosovich TY, Gray T et al (2004) Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 78(22):12665–12667. https://doi.org/10.1128/JVI.78.22.12665-12667.2004
Chen JD, Wang JH, Zhang JP et al (2021) Front Immunol 12:711997. https://doi.org/10.3389/fimmu.2021.711997
Huang K, Zhang TF, Gong WX et al (2021) PGRMC1 exerts its function of anti-influenza virus in the central nervous system. Microbiol Spectr 9(2):e0073421. https://doi.org/10.1128/Spectrum.00734-21
Jong MD, Bach VC, Phan TQ et al (2005) Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 352(7):686–691. https://doi.org/10.1056/NEJMoa044307
Bornand D, Toovey S, Jick SS et al (2016) The risk of new onset depression in association with influenza--A population-based observational study. Brain Behav Immun 53:131–137. https://doi.org/10.1016/j.bbi.2015.12.005
Zhang TT, Hong J, Di TT et al (2016) MPTP impairs dopamine D1 receptor-mediated survival of newborn neurons in ventral hippocampus to cause depressive-like behaviors in adult mice. Front Mol Neurosci 9:101. https://doi.org/10.3389/fnmol.2016.00101
Appel JR, Ye SY, Tang FL et al (2018) Increased microglial activity, impaired adult hippocampal neurogenesis, and depressive-like behavior in microglial VPS35-depleted mice. J Neurosci 38(26):5949–5968. https://doi.org/10.1523/JNEUROSCI.3621-17.2018
Zhou BT, Zhu ZQ, Ransom BR et al (2021) Oligodendrocyte lineage cells and depression. Mol Psychiatry 26(1):103–117. https://doi.org/10.1038/s41380-020-00930-0
Hosseini S, Wilk E, Michaelsen-Preusse K et al (2018) Long-term neuroinflammation induced by Influenza A virus infection and the impact on hippocampal neuron morphology and function. J Neurosci 38(12):3060–3080. https://doi.org/10.1523/JNEUROSCI.1740-17.2018
Hosseini S, Michaelsen-Preusse K, Schughart K et al (2021) Long-term consequence of non-neurotropic H3N2 influenza A virus infection for the progression of Alzheimer's disease symptoms. Front Cell Neurosci 15:643650. https://doi.org/10.3389/fncel.2021.643650
Jurgens HA, Amancherla K, Johnson RW (2012) Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci 32(12):3958–3968. https://doi.org/10.1523/JNEUROSCI.6389-11.2012
McAuley JL, Hornung F, Boyd KL et al (2007) Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe 2(4):240–249. https://doi.org/10.1016/j.chom.2007.09.001
Zheng M, Kanneganti TD (2020) The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev 297(1):26–38. https://doi.org/10.1111/imr.12909 Epub 2020 Jul 29
Boal-Carvalho I, Mazel-Sanchez B, Silva F et al (2020) Influenza A viruses limit NLRP3-NEK7-complex formation and pyroptosis in human macrophages. EMBO Rep 21(12):e50421. https://doi.org/10.15252/embr.202050421 Epub 2020 Nov 12
Zamarin D, García-Sastre A, Xiao XY et al (2005) Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog 1(1):e4. https://doi.org/10.1371/journal.ppat.0010004 Epub 2005 Sep 30
Jaworska J, Coulombe F, Downey J et al (2014) NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proc Natl Acad Sci USA 111(20):E2110–E2119. https://doi.org/10.1073/pnas.1322118111
Cen MY, Wei OY, Lin XH et al (2023) FBXO6 regulates the antiviral immune responses via mediating alveolar macrophages survival. J Med Virol 95(1):e28203. https://doi.org/10.1002/jmv.28203
Wang RF, Zhu YX, Ren CW et al (2021) Influenza A virus protein PB1-F2 impairs innate immunity by inducing mitophagy. Autophagy 17(2):496–511. https://doi.org/10.1080/15548627.2020.1725375
Arimori Y, Nakamura R, Yamada H et al (2013) Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antivir Res 99(3):230–237. https://doi.org/10.1016/j.antiviral.2013.05.007
Lee CH, Giuliani F (2019) The role of inflammation in depression and fatigue. Front Immunol 10:1696. https://doi.org/10.3389/fimmu.2019.01696
Suneson K, Lindahl J, Hårsmar SC et al (2021) Inflammatory depression-mechanisms and non-pharmacological interventions. Int J Mol Sci 22(4):1640. https://doi.org/10.3390/ijms22041640
Yang Q, Feng B, Zhang K et al (2012) Excessive astrocyte-derived neurotrophin-3 contributes to the abnormal neuronal dendritic development in a mouse model of fragile X syndrome. PLoS Genet 8(12):e1003172. https://doi.org/10.1371/journal.pgen.1003172
Hawes MT, Szenczy AK, Klein DN et al (2022) Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med 52(14):3222–3230. https://doi.org/10.1017/S0033291720005358
Ettman CK, Abdalla SM, Cohen GH et al (2020) Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open 3(9):e2019686. https://doi.org/10.1001/jamanetworkopen.2020.19686
Turk MC, Bakker CJ, Spencer SM et al (2022) Systematic review of sex differences in the relationship between hormones and depression in HIV. Psychoneuroendocrinology 138:105665. https://doi.org/10.1016/j.psyneuen.2022.105665
Adinolfi LE, Nevola R, Rinaldi L et al (2017) Chronic hepatitis C virus infection and depression. Clin Liver Dis 21(3):517–534. https://doi.org/10.1016/j.cld.2017.03.007
Umschweif G, Greengard P, Sagi Y (2021) The dentate gyrus in depression. Eur J Neurosci 53(1):39–64. https://doi.org/10.1111/ejn.14640
Jia XN, Gao ZH, Hu HL (2021) Microglia in depression: current perspectives. Sci China Life Sci 64(6):911–925. https://doi.org/10.1007/s11427-020-1815-6
Wang Q, Jie W, Liu JH et al (2017) An astroglial basis of major depressive disorder? An overview. Glia 65(8):1227–1250. https://doi.org/10.1002/glia.23143
Sun YX, Chen X, Ou ZM et al (2022) Dysmyelination by oligodendrocyte-specific ablation of Ninj2 contributes to depressive-like behaviors. Adv Sci (Weinh) 9(3):e2103065. https://doi.org/10.1002/advs.202103065
Kokkosis AG, Madeira MM, Mullahy MR et al (2022) Chronic stress disrupts the homeostasis and progeny progression of oligodendroglial lineage cells, associating immune oligodendrocytes with prefrontal cortex hypomyelination. Mol Psychiatry 27(6):2833–2848. https://doi.org/10.1038/s41380-022-01512-y
Boda E (2021) Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur J Neurosci 53(1):281–297. https://doi.org/10.1111/ejn.14621
Yazdankhah M, Shang P, Ghosh S et al (2021) Role of glia in optic nerve. Prog Retin Eye Res 81:100886. https://doi.org/10.1016/j.preteyeres.2020.100886
Xin W, Mironova YA, Shen H et al (2019) Oligodendrocytes support neuronal glutamatergic transmission via expression of glutamine synthetase. Cell Rep 27(8):2262-2271.e5. https://doi.org/10.1016/j.celrep.2019.04.094
Zhang M, Cheng XF, Dang RZ et al (2018) Lactate Deficit in an Alzheimer Disease Mouse Model: The Relationship With Neuronal Damage. J Neuropathol Exp Neurol 77(12):1163–1176. https://doi.org/10.1093/jnen/nly102
Pernet V, Joly S, Christ F et al (2008) Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci 28(29):7435–7444. https://doi.org/10.1523/JNEUROSCI.0727-08.2008
Schmandke A, Schmandke A, Schwab ME (2014) Nogo-A: multiple roles in CNS development, maintenance, and disease. Neuroscientist 20(4):372–386. https://doi.org/10.1177/1073858413516800
Delekate A, Zagrebelsky M, Kramer S et al (2011) NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci USA 108(6):2569–2574. https://doi.org/10.1073/pnas.1013322108
Acknowledgements
We thank the National Natural Science Foundation of China and the Shaanxi Province Key Industry Innovation Chain Project for funding.
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers 31972902, 81771469, and 31800887) and partially supported by the Shaanxi Province Key Industry Innovation Chain Project (Grant number 2023-ZDLSF-59).
Author information
Authors and Affiliations
Contributions
Le Yang, Rui Liu, and Peijun Han conceived and designed this study. Le Yang, Minggao Zhao and Gaofei Wei supervised this study. Saiying Wang, Haijun Zhang, Caiyan Cheng, Yue Chen, Zheng Rong, Fei Li, and Chang Su performed data collection and analysis. Saiying Wang and Le Yang drafted the manuscript. Qi Yang revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Ethics Committee of the Fourth Military Medical University (Approval No. KY20193145) in full compliance with the ethical guidelines of the National Institutes of Health for the care and use of laboratory animals.
Consent to Participate
Not applicable.
Consent for Publication
All authors have consented to publish in the journal Molecular Neurobiology.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Supplementary fig. s1 (DOCX 506 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Zhang, H., Liu, R. et al. Influenza A Virus PB1-F2 Induces Affective Disorder by Interfering Synaptic Plasticity in Hippocampal Dentate Gyrus. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04107-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04107-6