Abstract
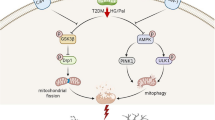
Diabetes-associated cognitive dysfunction (DACD) has ascended to become the second leading cause of mortality among diabetic patients. Phosphoserine phosphatase (PSPH), a pivotal rate-limiting enzyme in L-serine biosynthesis, has been documented to instigate the insulin signaling pathway through dephosphorylation. Concomitantly, CD38, acting as a mediator in mitochondrial transfer, is activated by the insulin pathway. Given that we have demonstrated the beneficial effects of exogenous mitochondrial supplementation on DACD, we further hypothesized whether astrocytic PSPH could contribute to improving DACD by promoting astrocytic mitochondrial transfer into neurons. In the Morris Water Maze (MWM) test, our results demonstrated that overexpression of PSPH in astrocytes alleviated DACD in db/db mice. Astrocyte specific-stimulated by PSPH lentivirus/ adenovirus promoted the spine density both in vivo and in vitro. Mechanistically, astrocytic PSPH amplified the expression of CD38 via initiation of the insulin signaling pathway, thereby promoting astrocytic mitochondria transfer into neurons. In summation, this comprehensive study delineated the pivotal role of astrocytic PSPH in alleviating DACD and expounded upon its intricate cellular mechanism involving mitochondrial transfer. These findings propose that the specific up-regulation of astrocytic PSPH holds promise as a discerning therapeutic modality for DACD.







Similar content being viewed by others
Data Availability
All data that support the fundings of this study are available from corresponding author upon reasonable request.
Abbreviations
- AAV:
-
Adeno-Associated Viruses
- ACM:
-
Astrocyte-Conditioned Media
- AKT:
-
Protein Kinase B
- ATP:
-
Adenosine Triphosphate
- cADPR:
-
cyclic ADP-Ribose
- CD38:
-
Cluster of Differentiation 38
- CO-IP:
-
Co-Immunoprecipitation
- DACD:
-
Diabetes-Associated Cognitive Dysfunction
- DMEM:
-
Dulbecco?s Modified Eagle Medium
- EDTA:
-
Ethylenediaminetetraacetic Acid
- GFAP:
-
Glial Fibrillary Acidic Protein
- GSK3?:
-
Glycogen Synthase Kinase-3?
- HE:
-
Haematoxylin and Eosin
- IF:
-
Immunofluorescent Staining
- IRS-1:
-
Insulin Receptor Substrate 1
- LV:
-
Lentivirus
- MAPK:
-
Mitogen-Activated Protein Kinase
- MWM:
-
Morris Water Maze
- PSPH:
-
Phosphoserine Phosphatase
- SDS-PAGE:
-
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
- TUNEL:
-
TdT-Mediated dUTP Nick-End Labeling
- T2DM:
-
Type 2 Diabetes Mellitus
References
Valencia WM, Florez H (2017) How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 356:i6505. https://doi.org/10.1136/bmj.i6505
Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT (2019) Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev 55:100944. https://doi.org/10.1016/j.arr.2019.100944
Biessels GJ, Whitmer RA (2020) Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia 63(1):3–9. https://doi.org/10.1007/s00125-019-04977-9
Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, Gregg EW (2021) Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 9(3):165–173. https://doi.org/10.1016/S2213-8587(20)30431-9
de Galan BE, Zoungas S, Chalmers J, Anderson C, Dufouil C, Pillai A, Cooper M, Grobbee DE, Hackett M, Hamet P, Heller SR, Lisheng L, MacMahon S, Mancia G, Neal B, Pan CY, Patel A, Poulter N, Travert F, Woodward M (2009) group AC Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 52 (11):2328–2336. https://doi.org/10.1007/s00125-009-1484-7
Holm LJ, Buschard K (2019) L-serine: a neglected amino acid with a potential therapeutic role in diabetes. APMIS 127(10):655–659. https://doi.org/10.1111/apm.12987
Park SM, Seo EH, Bae DH, Kim SS, Kim J, Lin W, Kim KH, Park JB, Kim YS, Yin J, Kim SY (2019) Phosphoserine Phosphatase promotes Lung Cancer Progression through the dephosphorylation of IRS-1 and a noncanonical L-Serine-independent pathway. Mol Cells 42(8):604–616. https://doi.org/10.14348/molcells.2019.0160
Kampen KR, Fancello L, Girardi T, Rinaldi G, Planque M, Sulima SO, Loayza-Puch F, Verbelen B, Vereecke S, Verbeeck J, Op de Beeck J, Royaert J, Vermeersch P, Cassiman D, Cools J, Agami R, Fiers M, Fendt SM, De Keersmaecker K (2019) Translatome analysis reveals altered serine and glycine metabolism in T-cell acute lymphoblastic leukemia cells. Nat Commun 10(1):2542. https://doi.org/10.1038/s41467-019-10508-2
Vincent JB, Jamil T, Rafiq MA, Anwar Z, Ayaz M, Hameed A, Nasr T, Naeem F, Khattak NA, Carter M, Ahmed I, John P, Wiame E, Andrade DM, Schaftingen EV, Mir A, Ayub M (2015) Phosphoserine phosphatase (PSPH) gene mutation in an intellectual disability family from Pakistan. Clin Genet 87(3):296–298. https://doi.org/10.1111/cge.12445
Begum N, Sussman KE, Draznin B (1992) Calcium-induced inhibition of phosphoserine phosphatase in insulin target cells is mediated by the phosphorylation and activation of inhibitor 1. J Biol Chem 267(9):5959–5963
Giovannoni F, Quintana FJ (2020) The role of astrocytes in CNS inflammation. Trends Immunol 41(9):805–819. https://doi.org/10.1016/j.it.2020.07.007
Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16(5):249–263. https://doi.org/10.1038/nrn3898
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504(7480):394–400. https://doi.org/10.1038/nature12776
Kreft M, Bak LK, Waagepetersen HS, Schousboe A (2012) Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro 4(3). https://doi.org/10.1042/AN20120007
Mills WA 3rd, Woo AM, Jiang S, Martin J, Surendran D, Bergstresser M, Kimbrough IF, Eyo UB, Sofroniew MV, Sontheimer H (2022) Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat Commun 13(1):1794. https://doi.org/10.1038/s41467-022-29475-2
Rahman MH, Bhusal A, Kim JH, Jha MK, Song GJ, Go Y, Jang IS, Lee IK, Suk K (2020) Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nat Commun 11(1):5906. https://doi.org/10.1038/s41467-020-19576-1
Gundersen V, Storm-Mathisen J, Bergersen LH (2015) Neuroglial Transmission. Physiol Rev 95(3):695–726. https://doi.org/10.1152/physrev.00024.2014
Tan Z, Liu Y, Xi W, Lou HF, Zhu L, Guo Z, Mei L, Duan S (2017) Glia-derived ATP inversely regulates excitability of pyramidal and CCK-positive neurons. Nat Commun 8:13772. https://doi.org/10.1038/ncomms13772
Koh W, Park M, Chun YE, Lee J, Shim HS, Park MG, Kim S, Sa M, Joo J, Kang H, Oh SJ, Woo J, Chun H, Lee SE, Hong J, Feng J, Li Y, Ryu H, Cho J, Lee CJ (2022) Astrocytes render memory flexible by releasing D-Serine and regulating NMDA receptor tone in the Hippocampus. Biol Psychiatry 91(8):740–752. https://doi.org/10.1016/j.biopsych.2021.10.012
Cheung G, Bataveljic D, Visser J, Kumar N, Moulard J, Dallerac G, Mozheiko D, Rollenhagen A, Ezan P, Mongin C, Chever O, Bemelmans AP, Lubke J, Leray I, Rouach N (2022) Physiological synaptic activity and recognition memory require astroglial glutamine. Nat Commun 13(1):753. https://doi.org/10.1038/s41467-022-28331-7
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. https://doi.org/10.1038/nature18928
Amin SN (2021) Platelets: the peripheral donor of mitochondria for diabetes-induced cognitive impairment. Clin Sci (Lond) 135(4):593–595. https://doi.org/10.1042/CS20201297
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503. https://doi.org/10.1016/j.redox.2018.01.008
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G, Gao W, Li Y, Wang Q (2020) Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci (Lond) 134(16):2161–2175. https://doi.org/10.1042/CS20200530
Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, Kay KR, Berrocoso E, Murillo-Carretero MI, Perdomo G, Spires-Jones T, Cozar-Castellano I, Lechuga-Sancho AM, Garcia-Alloza M (2013) Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 38(11):2462–2475. https://doi.org/10.1016/j.psyneuen.2013.05.010
Luo Q, Xian P, Wang T, Wu S, Sun T, Wang W, Wang B, Yang H, Yang Y, Wang H, Liu W, Long Q (2021) Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics 11(12):5986–6005. https://doi.org/10.7150/thno.58632
Yamasaki M, Yamada K, Furuya S, Mitoma J, Hirabayashi Y, Watanabe M (2001) 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J Neurosci 21(19):7691–7704. https://doi.org/10.1523/JNEUROSCI.21-19-07691.2001
Wolosker H, Radzishevsky I (2013) The serine shuttle between glia and neurons: implications for neurotransmission and neurodegeneration. Biochem Soc Trans 41(6):1546–1550. https://doi.org/10.1042/BST20130220
Akhtar MW, Sanz-Blasco S, Dolatabadi N, Parker J, Chon K, Lee MS, Soussou W, McKercher SR, Ambasudhan R, Nakamura T, Lipton SA (2016) Elevated glucose and oligomeric beta-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat Commun 7:10242. https://doi.org/10.1038/ncomms10242
Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP (2009) Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19(10):951–961. https://doi.org/10.1002/hipo.20577
von Bohlen Und Halbach O (2009) Structure and function of dendritic spines within the hippocampus. Ann Anat 191(6):518–531. https://doi.org/10.1016/j.aanat.2009.08.006
Shawl AI, Park KH, Kim UH (2009) Insulin receptor signaling for the proliferation of pancreatic beta-cells: involvement of Ca2 + second messengers, IP3, NAADP and cADPR. Islets 1(3):216–223. https://doi.org/10.4161/isl.1.3.9646
Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, Nonaka Y, Okamoto M (2003) Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J Biol Chem 278(20):18440–18447. https://doi.org/10.1074/jbc.M211770200
Tzatsos A, Tsichlis PN (2007) Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem 282(25):18069–18082. https://doi.org/10.1074/jbc.M610101200
Bervoets L, Massa G, Guedens W, Louis E, Noben JP, Adriaensens P (2017) Metabolic profiling of type 1 diabetes mellitus in children and adolescents: a case-control study. Diabetol Metab Syndr 9:48. https://doi.org/10.1186/s13098-017-0246-9
Bertea M, Rutti MF, Othman A, Marti-Jaun J, Hersberger M, von Eckardstein A, Hornemann T (2010) Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis 9:84. https://doi.org/10.1186/1476-511X-9-84
Suzuki M, Sasabe J, Furuya S, Mita M, Hamase K, Aiso S (2012) Type 1 diabetes mellitus in mice increases hippocampal D-serine in the acute phase after streptozotocin injection. Brain Res 1466:167–176. https://doi.org/10.1016/j.brainres.2012.05.042
Wang Y, Ni J, Gao T, Gao C, Guo L, Yin X (2020) Activation of astrocytic sigma-1 receptor exerts antidepressant-like effect via facilitating CD38-driven mitochondria transfer. Glia 68(11):2415–2426. https://doi.org/10.1002/glia.23850
Holscher C (2020) Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs 29(4):333–348. https://doi.org/10.1080/13543784.2020.1738383
Kellar D, Craft S (2020) Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 19(9):758–766. https://doi.org/10.1016/S1474-4422(20)30231-3
Xu J, Gao H, Zhang L, Rong S, Yang W, Ma C, Chen M, Huang Q, Deng Q, Huang F (2019) Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J Pineal Res 67(2):e12584. https://doi.org/10.1111/jpi.12584
Cai W, Xue C, Sakaguchi M, Konishi M, Shirazian A, Ferris HA, Li ME, Yu R, Kleinridders A, Pothos EN, Kahn CR (2018) Insulin regulates astrocyte gliotransmission and modulates behavior. J Clin Invest 128(7):2914–2926. https://doi.org/10.1172/JCI99366
Yamamoto N, Matsubara T, Sobue K, Tanida M, Kasahara R, Naruse K, Taniura H, Sato T, Suzuki K (2012) Brain insulin resistance accelerates Abeta fibrillogenesis by inducing GM1 ganglioside clustering in the presynaptic membranes. J Neurochem 121(4):619–628. https://doi.org/10.1111/j.1471-4159.2012.07668.x
Girault FM, Sonnay S, Gruetter R, Duarte JMN (2019) Alterations of Brain Energy metabolism in type 2 Diabetic Goto-Kakizaki rats measured in vivo by (13)C magnetic resonance spectroscopy. Neurotox Res 36(2):268–278. https://doi.org/10.1007/s12640-017-9821-y
Durkee CA, Araque A (2019) Diversity and specificity of astrocyte-neuron communication. Neuroscience 396:73–78. https://doi.org/10.1016/j.neuroscience.2018.11.010
Herrera Moro Chao D, Kirchner MK, Pham C, Foppen E, Denis RGP, Castel J, Morel C, Montalban E, Hassouna R, Bui LC, Renault J, Mouffle C, Garcia-Caceres C, Tschop MH, Li D, Martin C, Stern JE, Luquet SH (2022) Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab 34(10):1532–1547e1536. https://doi.org/10.1016/j.cmet.2022.09.002
Shima T, Jesmin S, Matsui T, Soya M, Soya H (2018) Differential effects of type 2 diabetes on brain glycometabolism in rats: focus on glycogen and monocarboxylate transporter 2. J Physiol Sci 68(1):69–75. https://doi.org/10.1007/s12576-016-0508-6
Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD (2010) Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab 30(8):1527–1537. https://doi.org/10.1038/jcbfm.2010.61
Funding
National Natural Science Foundation of China (Grants Nos. 81974540, 81801899), the Key Research & Development Program of Shaanxi (Program No. 2022ZDLSF02-09) and Innovation Capability Support Program of Shaanxi (Program No. 2021TD-58), Basic Research Program of Natural Sciences of Shaanxi Province (Grant No. 2022JQ-850).
Author information
Authors and Affiliations
Contributions
Q.W., J.Z., H.M., and Y.L. contributed to the conceptualization and study design. Development of methodology, investigation, and original draft preparation was done by H.M., S.H., Y.L., X.Z., H.C., M.D., S.J., H.G., and C.Y. assisted in experimental procedures. Manuscript review and editing was done by H.M., S.H., S.J., H.G., Q.W., J.Z. All the authors read and approved the final manuscript. H.M. and S.H. contributed equally to this study.
Corresponding authors
Ethics declarations
Ethical Approval
All experiments involving animals have obtained approval from the Institutional Animal Care and Use Committees of Xi’an Jiaotong University (NO. 2019-060).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, H., He, S., Li, Y. et al. Augmented Mitochondrial Transfer Involved in Astrocytic PSPH Attenuates Cognitive Dysfunction in db/db Mice. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04064-0