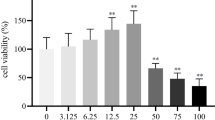
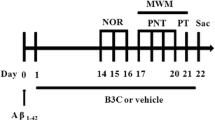
Abstract
Bisphenol A (BPA) is a component of polycarbonate plastics that has been implicated in memory impairment. The present study investigated the effect of carnosic acid (CA) on memory deficit induced by BPA and the role of Akt in this mechanism. First, SH-SY5Y cells were treated with 20 nM BPA and 1 μM CA for 12 h. The results showed that treatment of CA with BPA improved the alternation of IRS-1/Akt/GSK-3β as well as the induction of ApoE and Ser396p-tau. Moreover, treatment of CA with BPA restored the signaling involved in long-term potentiation (LTP) effect, leading to induction of synaptic-related proteins, such as PSD-95, synapsin1a, and pro-BDNF. Wortmannin treatment alleviated the reversal by CA. Then, C57BL/6 J male mice were orally administered with CA to test the memory function in BPA treatment. The results showed that CA and RE can improve BPA-induced impairment of motor, recognition, and spatial memory by using open-field test (OFT), novel objective recognition test (NOR), and Y-maze test, respectively. Moreover, CA and RE improved the phosphorylation of tau and the reduction of PSD-95, synapsin1a, and pro-BDNF proteins induced by BPA. Therefore, the results indicated that CA decreased the phosphorylated tau and memory impairment induced by BPA through Akt pathway.







Similar content being viewed by others
Data Availability
Data is available on request from the authors.
Abbreviations
- AD:
-
Alzheimer’s disease
- Akt:
-
Protein kinase B
- ApoE:
-
Apolipoprotein E
- Aβ:
-
Amyloid beta
- BDNF:
-
Brain-derived neurotrophic factor
- BPA:
-
Bisphenol A
- CA:
-
Carnosic acid
- CREB:
-
CAMP response element-binding protein
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DMSO:
-
Dimethyl sulfoxide
- ERK:
-
Extracellular signal-regulated kinase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GSK:
-
Glycogen synthase kinase
- HRP:
-
Horseradish peroxidase
- IB:
-
Immunoblotting
- IP assay:
-
Immunoprecipitation assay
- IRS-1:
-
Insulin response sequence-1
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolim bromide
- NOR:
-
Novel object recognition test
- OFT:
-
Open-field test
- PSD-95:
-
Post-synaptic density protein 95
- RA:
-
Retinoic acid
References
Wu D, Wu F, Lin R, Meng Y, Wei W, Sun Q, Jia L (2020) Impairment of learning and memory induced by perinatal exposure to BPA is associated with ERalpha-mediated alterations of synaptic plasticity and PKC/ERK/CREB signaling pathway in offspring rats. Brain Res Bull 161:43–54. https://doi.org/10.1016/j.brainresbull.2020.04.023
Hoekstra EJ, Simoneau C (2013) Release of bisphenol A from polycarbonate: a review. Crit Rev Food Sci Nutr 53(4):386–402. https://doi.org/10.1080/10408398.2010.536919
Nesan D, Sewell LC, Kurrasch DM (2018) Opening the black box of endocrine disruption of brain development: lessons from the characterization of Bisphenol A. Horm Behav 101:50–58. https://doi.org/10.1016/j.yhbeh.2017.12.001
EFSA Panel on Food Contact Materials E, Processing A, Lambre C, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R et al (2023) Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 21(4):e06857. https://doi.org/10.2903/j.efsa.2023.6857
Akash MSH, Sabir S, Rehman K (2020) Bisphenol A-induced metabolic disorders: from exposure to mechanism of action. Environ Toxicol Pharmacol 77:103373. https://doi.org/10.1016/j.etap.2020.103373
Cimmino I, Fiory F, Perruolo G, Miele C, Beguinot F, Formisano P, Oriente F (2020) Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int J Mol Sci 21(16):5761. https://doi.org/10.3390/ijms21165761
Wang T, Xie C, Yu P, Fang F, Zhu J, Cheng J, Gu A, Wang J et al (2017) Involvement of insulin signaling disturbances in bisphenol A-induced Alzheimer’s disease-like neurotoxicity. Sci Rep 7(1):7497. https://doi.org/10.1038/s41598-017-07544-7
Li J, Wang Y, Fang F, Chen D, Gao Y, Liu J, Gao R, Wang J et al (2016) Bisphenol A disrupts glucose transport and neurophysiological role of IR/IRS/AKT/GSK3beta axis in the brain of male mice. Environ Toxicol Pharmacol 43:7–12. https://doi.org/10.1016/j.etap.2015.11.025
Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB (2016) Insulin in the nervous system and the mind: functions in metabolism, memory, and mood. Mol Metab 5(8):589–601. https://doi.org/10.1016/j.molmet.2016.06.011
Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B (2013) Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 126:43–50. https://doi.org/10.1016/j.envres.2013.06.004
Yin Z, Hua L, Chen L, Hu D, Li J, An Z, Tian T, Ning H et al (2020) Bisphenol-A exposure induced neurotoxicity and associated with synapse and cytoskeleton in Neuro-2a cells. Toxicol In Vitro 67:104911. https://doi.org/10.1016/j.tiv.2020.104911
Hong SB, Hong YC, Kim JW, Park EJ, Shin MS, Kim BN, Yoo HJ, Cho IH et al (2013) Bisphenol A in relation to behavior and learning of school-age children. J Child Psychol Psychiatry 54(8):890–899. https://doi.org/10.1111/jcpp.12050
Chen Z, Li T, Zhang L, Wang H, Hu F (2018) Bisphenol A exposure remodels cognition of male rats attributable to excitatory alterations in the hippocampus and visual cortex. Toxicology 410:132–141. https://doi.org/10.1016/j.tox.2018.10.002
Zhou Y, Wang Z, Xia M, Zhuang S, Gong X, Pan J, Li C, Fan R et al (2017) Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: implications for children exposed to environmental levels of BPA. Environ Pollut 229:40–48. https://doi.org/10.1016/j.envpol.2017.05.043
Xu X, Liu X, Zhang Q, Zhang G, Lu Y, Ruan Q, Dong F, Yang Y (2013) Sex-specific effects of bisphenol-A on memory and synaptic structural modification in hippocampus of adult mice. Horm Behav 63(5):766–775. https://doi.org/10.1016/j.yhbeh.2013.03.004
Lai CY, Lin CY, Wu CR, Tsai CH, Tsai CW (2021) Carnosic acid alleviates levodopa-induced dyskinesia and cell death in 6-hydroxydopamine-lesioned rats and in SH-SY5Y cells. Front Pharmacol 12:703894. https://doi.org/10.3389/fphar.2021.703894
Azad N, Rasoolijazi H, Joghataie MT, Soleimani S (2011) Neuroprotective effects of carnosic acid in an experimental model of Alzheimer’s disease in rats. Cell J 13(1):39–44
Teppola H, Sarkanen JR, Jalonen TO, Linne ML (2016) Morphological differentiation towards neuronal phenotype of SH-SY5Y neuroblastoma cells by estradiol, retinoic acid and cholesterol. Neurochem Res 41(4):731–747. https://doi.org/10.1007/s11064-015-1743-6
Chen YF, Lu YH, Tsai HY (2022) Crude extract of Desmodium gangeticum process anticancer activity via arresting cell cycle in G1 and modulating cell cycle-related protein expression in A549 human lung carcinoma cells. Biomedicine (Taipei) 12(2):31–39. https://doi.org/10.37796/2211-8039.1362
Lin CY, Tsai CW (2019) PINK1/parkin-mediated mitophagy pathway is related to neuroprotection by carnosic acid in SH-SY5Y cells. Food Chem Toxicol 125:430–437. https://doi.org/10.1016/j.fct.2019.01.027
Lin CY, Tsai CW (2017) Carnosic acid attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells by inducing autophagy through an enhanced interaction of Parkin and Beclin1. Mol Neurobiol 54(4):2813–2822. https://doi.org/10.1007/s12035-016-9873-7
Seibenhener ML, Wooten MC (2015) Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Jove-J Vis Exp 96:e52434. https://doi.org/10.3791/52434
Zhang R, Xue G, Wang S, Zhang L, Shi C, Xie X (2012) Novel object recognition as a facile behavior test for evaluating drug effects in AbetaPP/PS1 Alzheimer’s disease mouse model. J Alzheimers Dis 31(4):801–812. https://doi.org/10.3233/JAD-2012-120151
Belo RF, Martins MLF, Shvachiy L, Costa-Coelho T, de Almeida-Borlido C, Fonseca-Gomes J, Neves V, Vicente Miranda H et al (2020) The neuroprotective action of amidated-kyotorphin on amyloid beta peptide-induced Alzheimer’s disease pathophysiology. Front Pharmacol 11:985. https://doi.org/10.3389/fphar.2020.00985
Wang H, Xu J, Lazarovici P, Quirion R, Zheng W (2018) cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosci 11:255. https://doi.org/10.3389/fnmol.2018.00255
Wang C, Li Z, Han H, Luo G, Zhou B, Wang S, Wang J (2016) Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of Akt and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology 341–343:56–64. https://doi.org/10.1016/j.tox.2016.01.010
Chen L, Lau AG, Sarti F (2014) Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology 78:3–12. https://doi.org/10.1016/j.neuropharm.2012.12.004
Guo J, Walss-Bass C, Luduena RF (2010) The beta isotypes of tubulin in neuronal differentiation. Cytoskeleton (Hoboken) 67(7):431–441. https://doi.org/10.1002/cm.20455
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1–3):3–33. https://doi.org/10.1016/s0014-2999(03)01272-x
Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. https://doi.org/10.1016/j.bbr.2014.08.002
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916:105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) (2010) Scientific Opinion on Bisphenol A: evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of Bisphenol A. EFSA J 8:1829. https://doi.org/10.2903/j.efsa.2010.1829
Chen Z, Zuo X, He D, Ding S, Xu F, Yang H, Jin X, Fan Y et al (2017) Long-term exposure to a ‘safe’ dose of bisphenol A reduced protein acetylation in adult rat testes. Sci Rep 7:40337. https://doi.org/10.1038/srep40337
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) (2015) Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 13:3978. https://doi.org/10.2903/j.efsa.2015.3978
Xue J, Zhang L, Xie X, Gao Y, Jiang L, Wang J, Wang Y, Gao R et al (2020) Prenatal bisphenol A exposure contributes to Tau pathology: potential roles of CDK5/GSK3beta/PP2A axis in BPA-induced neurotoxicity. Toxicology 438:152442. https://doi.org/10.1016/j.tox.2020.152442
Zhang H, Kuang H, Luo Y, Liu S, Meng L, Pang Q, Fan R (2019) Low-dose bisphenol A exposure impairs learning and memory ability with alterations of neuromorphology and neurotransmitters in rats. Sci Total Environ 697:134036. https://doi.org/10.1016/j.scitotenv.2019.134036
Galizzi G, Di Carlo M (2022) Insulin and its key role for mitochondrial function/dysfunction and quality control: a shared link between dysmetabolism and neurodegeneration. Biology (Basel) 11(6):943. https://doi.org/10.3390/biology11060943
Fang F, Gao Y, Wang T, Chen D, Liu J, Qian W, Cheng J, Gao R et al (2016) Insulin signaling disruption in male mice due to perinatal bisphenol A exposure: role of insulin signaling in the brain. Toxicol Lett 245:59–67. https://doi.org/10.1016/j.toxlet.2016.01.007
Metaxas A, Kempf SJ (2016) Neurofibrillary tangles in Alzheimer’s disease: elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen Res 11(10):1579–1581. https://doi.org/10.4103/1673-5374.193234
Rhea EM, Raber J, Banks WA (2020) ApoE and cerebral insulin: trafficking, receptors, and resistance. Neurobiol Dis 137:104755. https://doi.org/10.1016/j.nbd.2020.104755
Wang Z, Alderman MH, Asgari C, Taylor HS (2020) Fetal bisphenol-A induced changes in murine behavior and brain gene expression persisted in adult-aged offspring. Endocrinology 161(12):bqaa164. https://doi.org/10.1210/endocr/bqaa164
Luo M, Li L, Ding M, Niu Y, Xu X, Shi X, Shan N, Qiu Z et al (2023) Long-term potentiation and depression regulatory microRNAs were highlighted in bisphenol A induced learning and memory impairment by microRNA sequencing and bioinformatics analysis. PLoS One 18(1):e0279029. https://doi.org/10.1371/journal.pone.0279029
El Morsy EM, Ahmed M (2020) Protective effects of lycopene on hippocampal neurotoxicity and memory impairment induced by bisphenol A in rats. Hum Exp Toxicol 39(8):1066–1078. https://doi.org/10.1177/0960327120909882
Rasoolijazi H, Azad N, Joghataei MT, Kerdari M, Nikbakht F, Soleimani M (2013) The protective role of carnosic acid against beta-amyloid toxicity in rats. Sci World J 2013:917082. https://doi.org/10.1155/2013/917082
Magee JC, Grienberger C (2020) Synaptic plasticity forms and functions. Annu Rev Neurosci 43:95–117. https://doi.org/10.1146/annurev-neuro-090919-022842
Wang C, Niu R, Zhu Y, Han H, Luo G, Zhou B, Wang J (2014) Changes in memory and synaptic plasticity induced in male rats after maternal exposure to bisphenol A. Toxicology 322:51–60. https://doi.org/10.1016/j.tox.2014.05.001
Funding
This study was supported by the Ministry of Science and Technology (MOST 110–2320-B-039–050-MY3) and the China Medical University (CMU111-MF-77 and CMU112-MF-68).
Author information
Authors and Affiliations
Contributions
Shaoi Hsu and Ruhuei Fu contributed equally to this work. Data curation and validation were performed by Shaoi Hsu, Huichi Huang, Chunhuei Liao, Hsiyun Huang, Yachen Shih, Jingwei Chen, Hanting Wu, and Tzuyu Kuo. The first draft of the manuscript was written by Shaoi Hsu. Ruhuei Fu performed conceptualization, methodology, resources, and supervision. Chiawen Tsai conceived the project, funding acquisition, project administration, resources, supervision, and writing the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All animal procedures were evaluated and approved by the Institutional Animal Care and Use Committee of China Medical University (protocol no. CMUIACUC-2021–099-1).
Consent for Publication
All listed authors have approved the final manuscript before submission, including the names and order of authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• BPA induced the alternation of insulin signaling and synaptic plasticity in SH-SY5Y cells and mice.
• BPA exposure caused accumulation of p-tau and apo E.
• BPA exposure impairs recognition and memory ability in mice.
• CA and RE improved BPA-induced phosphorylated tau and memory impairment.
• CA and RE prevented BPA-induced adverse effects through Akt pathway.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsu, S., Huang, H., Liao, C. et al. Induction of Phosphorylated Tau Accumulation and Memory Impairment by Bisphenol A and the Protective Effects of Carnosic Acid in In Vitro and In Vivo. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03952-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03952-9