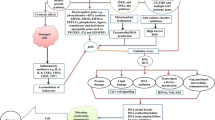
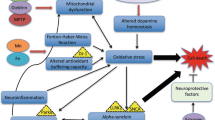
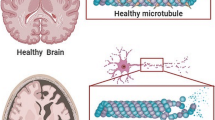
Abstract
Alzheimer’s disease is a leading cause of mortality worldwide. Inorganic and organic hazards, susceptibility to harmful metals, pesticides, agrochemicals, and air pollution are major environmental concerns. As merely 5% of AD cases are directly inherited indicating that these environmental factors play a major role in disease development. Long-term exposure to environmental toxins is believed to progress neuropathology, which leads to the development of AD. Numerous in-vitro and in-vivo studies have suggested the harmful impact of environmental toxins at cellular and molecular level. Common mechanisms involved in the toxicity of these environmental pollutants include oxidative stress, neuroinflammation, mitochondrial dysfunction, abnormal tau, and APP processing. Increased expression of GSK-3β, BACE-1, TNF-α, and pro-apoptotic molecules like caspases is observed upon exposure to these environmental toxins. In addition, the expression of neurotrophins like BDNF and GAP-43 have been found to be reduced as a result of toxicity. Further, modulation of signaling pathways involving PARP-1, PGC-1α, and MAPK/ERK induced by toxins have been reported to contribute in AD pathogenesis. These pathways are a promising target for developing novel AD therapeutics. Drugs like epigallocatechin-gallate, neflamapimod, salsalate, dexmedetomidine, and atabecestat are in different phases of clinical trials targeting the pathways for possible treatment of AD. This review aims to culminate the correlation between environmental toxicants and AD development. We emphasized upon the signaling pathways involved in the progression of the disease and the therapeutics under clinical trial targeting the altered pathways for possible treatment of AD.




Similar content being viewed by others
Data Availability
Not applicable.
References
Dhapola R, Sarma P, Medhi B, et al. (2021) Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer’s disease. Mol Neurobiol 1–21
Bhatti JS, Kaur S, Mishra J et al (2023) Targeting dynamin-related protein-1 as a potential therapeutic approach for mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys acta Mol basis Dis 1869:166798. https://doi.org/10.1016/j.bbadis.2023.166798
Nagar P, Sharma P, Dhapola R, et al. (2023) Endoplasmic reticulum stress in Alzheimer’s disease: molecular mechanisms and therapeutic prospects. Life Sci 121983 https://doi.org/10.1016/J.LFS.2023.121983
Beura SK, Dhapola R, Panigrahi AR et al (2023) Antiplatelet drugs: potential therapeutic options for the management of neurodegenerative diseases. Med Res Rev 43:1835–1877. https://doi.org/10.1002/MED.21965
Beura SK, Dhapola R, Panigrahi AR et al (2022) Redefining oxidative stress in Alzheimer’s disease: targeting platelet reactive oxygen species for novel therapeutic options. Life Sci 306:120855. https://doi.org/10.1016/J.LFS.2022.120855
Kumari S, Dhapola R, Reddy DHK (2023) Apoptosis in Alzheimer’s disease: insight into the signaling pathways and therapeutic avenues. Apoptosis 1–15 https://doi.org/10.1007/S10495-023-01848-Y/FIGURES/3
Thakur S, Dhapola R, Sarma P, et al. (2022) Neuroinflammation in Alzheimer’s disease: current progress in molecular signaling and therapeutics. Inflammation 1–17 https://doi.org/10.1007/S10753-022-01721-1/TABLES/1
Dhapola R, Subhendu Hota S et al (2021) Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29:1669–1681. https://doi.org/10.1007/S10787-021-00889-6
Penney J, Ralvenius W, Psychiatry LT (2020) Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry 25:148–167. https://doi.org/10.1038/s41380-019-0468-3
Rahman MA, Rahman MS, Uddin MJ et al (2020) Emerging risk of environmental factors: insight mechanisms of Alzheimer’s diseases. Environ Sci Pollut Res 27:44659–44672
Lee HJ, Park MK, Seo YR (2018) Pathogenic mechanisms of heavy metal induced-Alzheimer’s disease. Toxicol Environ Health Sci 10:1–10. https://doi.org/10.1007/s13530-018-0340-x
Cacciottolo M, Wang X, Driscoll I et al (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7:e1022–e1022. https://doi.org/10.1038/tp.2016.280
Croze ML, Zimmer L (2018) Ozone atmospheric pollution and Alzheimer’s disease: from epidemiological facts to molecular mechanisms. J Alzheimer’s Dis 62:503–522. https://doi.org/10.3233/JAD-170857
Aloizou A, Siokas V, Vogiatzi C et al (2020) Pesticides, cognitive functions and dementia: a review. Toxicol Lett 326:31–51. https://doi.org/10.1016/j.toxlet.2020.03.005
Harikrishnareddy D, Misra S, Upadhyay S et al (2015) Roots to start research in amyotrophic lateral sclerosis: molecular pathways and novel therapeutics for future. Rev Neurosci 26:161–181. https://doi.org/10.1515/revneuro-2014-0057
Eid A, Mhatre I, Richardson JR (2019) Gene-environment interactions in Alzheimer’s disease: a potential path to precision medicine. Pharmacol Ther 199:173–187
Vasefi M, Ghaboolian-Zare E, Abedelwahab H, Osu A (2020) Environmental toxins and Alzheimer’s disease progression. Neurochem Int 141:104852
Pletz J, Sánchez-Bayo F, Tennekes HA (2016) Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury—implications for toxic effects in the developing brain. Toxicology 347–349:1–5. https://doi.org/10.1016/j.tox.2016.02.006
Masoud AM, Bihaqi SW, Machan JT et al (2016) Early-life exposure to lead (Pb) alters the expression of microRNA that target proteins associated with Alzheimer’s disease. J Alzheimers Dis 51:1257–1264. https://doi.org/10.3233/JAD-151018
Dunn AR, O’Connell KMS, Kaczorowski CC (2019) Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci Biobehav Rev 103:73–80. https://doi.org/10.1016/j.neubiorev.2019.06.018
Calderón-Garcidueñas L, de la Monte SM (2017) Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: recipe for Alzheimer’s disease development in Mexico City young females. J Alzheimers Dis 58:613–630. https://doi.org/10.3233/JAD-161299
Al-Gubory KH (2014) Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod Biomed Online 29:17–31. https://doi.org/10.1016/j.rbmo.2014.03.002
Ayyalasomayajula N, Bandaru M, PD, (2020) Inactivation of GAP-43 due to the depletion of cellular calcium by the Pb and amyloid peptide induced toxicity: an in vitro approach. Chem Biol Interact 316:108927. https://doi.org/10.1016/j.cbi.2019.108927
Khalid M, Abdollahi M (2019) Epigenetic modifications associated with pathophysiological effects of lead exposure. J Environ Sci Heal - Part C Environ Carcinog Ecotoxicol Rev 37:235–287. https://doi.org/10.1080/10590501.2019.1640581
Wu S, Liu H, Zhao H et al (2020) Environmental lead exposure aggravates the progression of Alzheimer’s disease in mice by targeting on blood brain barrier. Elsevier 319:138–147. https://doi.org/10.1016/j.toxlet.2019.11.009
Mir RH, Sawhney G, Pottoo FH et al (2020) Role of environmental pollutants in Alzheimer’s disease: a review. Environ Sci Pollut Res 27:44724–44742. https://doi.org/10.1007/s11356-020-09964-x
Bihaqi S, Eid A, Neurotoxicology NZ (2017) U (2017) Lead exposure and tau hyperphosphorylation: an in vitro study. Neurotoxicology 62:218–223
Hernández-Zimbrón LF, Rivas-Arancibia S (2015) Oxidative stress caused by ozone exposure induces β-amyloid 1–42 overproduction and mitochondrial accumulation by activating the amyloidogenic pathway. Neuroscience 304:340–348. https://doi.org/10.1016/j.neuroscience.2015.07.011
Rivas-Arancibia S, Rodríguez-Martínez E, Badillo-Ramírez I et al (2017) Structural changes of amyloid beta in hippocampus of rats exposed to ozone: a Raman spectroscopy study. Front Mol Neurosci 10:137. https://doi.org/10.3389/fnmol.2017.00137
Rodríguez-Martínez E, Nava-Ruiz C, Escamilla-Chimal E et al (2016) The effect of chronic ozone exposure on the activation of endoplasmic reticulum stress and apoptosis in rat hippocampus. Front Aging Neurosci 8:245. https://doi.org/10.3389/fnagi.2016.00245
Shou Y, Huang Y, Zhu X et al (2019) A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol Environ Saf 174:344–352. https://doi.org/10.1016/J.ECOENV.2019.02.086
Underwood E (2017) The polluted brain. Science 355(6323):342–345. https://doi.org/10.1126/science.355.6323
Cacciottolo M, Wang X, Driscoll I, et al. (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. nature.com
Oudin A, Segersson D, Adolfsson R, Forsberg B (2018) Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS One 13:e0198283. https://doi.org/10.1371/journal.pone.0198283
Younan D, Petkus A, Widaman K, Brain XW (2020) Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 143:289–302. https://doi.org/10.1093/brain/awz348
Martire S, Mosca L, d’Erme M (2015) PARP-1 involvement in neurodegeneration: a focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev 146–148:53–64. https://doi.org/10.1016/J.MAD.2015.04.001
Jang S, Kim EW, Zhang Y et al (2018) Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer’s mouse: involvement of PARP-1. Biochem Biophys Res Commun 500:333–338. https://doi.org/10.1016/J.BBRC.2018.04.068
Spangenberg E, Brain KG-, Behavior U et al (2017) Inflammation in Alzheimer’s disease: lessons learned from microglia-depletion models. Elsevier 61:1–11. https://doi.org/10.1016/j.bbi.2016.07.003
Hsu H-W, Bondy SC, Kitazawa M (2018) Environmental and dietary exposure to copper and its cellular mechanisms linking to Alzheimer’s disease. Toxicol Sci 163:338–345. https://doi.org/10.1093/toxsci/kfy025
Mathys ZK, White AR (2017) Copper and Alzheimer’s disease. Advances in Neurobiology. Springer, New York LLC, pp 199–216
Kaur S, Raj K, Gupta YK, Singh S (2021) Allicin ameliorates aluminium- and copper-induced cognitive dysfunction in Wistar rats: relevance to neuro-inflammation, neurotransmitters and Aβ((1–42)) analysis. J Biol Inorg Chem 26:495–510. https://doi.org/10.1007/s00775-021-01866-8
Sensi SL, Granzotto A, Siotto M, Squitti R (2018) Copper and zinc dysregulation in Alzheimer’s disease. 39:1049–1063 https://doi.org/10.1016/j.tips.2018.10.001
Elseweidy MM, Mahrous M, Sousou et al (2023) Pentoxifylline as add-on treatment to donepezil in copper sulphate-induced Alzheimer’s disease-like neurodegeneration in rats. Neurotox Res 2023(1):1–13. https://doi.org/10.1007/S12640-023-00672-1
Tang BL (2020) Neuropathological mechanisms associated with pesticides in Alzheimer’s disease. Toxics 8 https://doi.org/10.3390/toxics8020021
Medehouenou TCM, Ayotte P, Carmichael PH, et al. (2019) Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: a prospective analysis from the Canadian Study of Health and Aging. Environ Heal A Glob Access Sci Source 18 https://doi.org/10.1186/s12940-019-0494-2
Schmidt S (2020) Fungicide exposure and amyloid plaques in mice: further evidence of an environmental risk factor for Alzheimer’s disease. ehp.niehs.nih.gov 128:1–2 https://doi.org/10.1289/EHP7021
Lafon P-A, Arango-Lievano M, Salvador-Prince L et al (2020) Fungicide residues exposure and β-amyloid aggregation in a mouse model of Alzheimer’s disease. Environ Health Perspect 128:17011. https://doi.org/10.1289/EHP5550
Junio J, Branca V, Maresca M et al (2019) Effects of cadmium on ZO-1 tight junction integrity of the blood brain barrier. Int J Mol Sci 20:6010. https://doi.org/10.3390/ijms20236010
Branca JJV, Morucci G, Pacini A (2018) Cadmium-induced neurotoxicity: still much ado. Neural Regen Res 13:1879–1882
Zhang L, Wang H, Abel G et al (2020) The effects of gene-environment interactions between cadmium exposure and apolipoprotein E4 on memory in a mouse model of Alzheimer’s disease. Toxicol Sci 173:189–201. https://doi.org/10.1093/TOXSCI/KFZ218
Vasefi M, Ghabolian-Zare E, … HA-N, 2020 U (2020) Environmental toxins and Alzheimer’s disease progression. Neurochem Int 141:104852
Colomina MT, Peris-Sampedro F (2017) Aluminum and Alzheimer’s disease. Advances in Neurobiology. Springer, New York LLC, pp 183–197
Bondy SC (2016) Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 52:222–229
Nie J (2018) Exposure to aluminum in daily life and Alzheimer’s disease. Advances in Experimental Medicine and Biology. Springer, New York LLC, pp 99–111
Yin S, Ran Q, Yang J, et al. (2020) Nootropic effect of neferine on aluminium chloride–induced Alzheimer’s disease in experimental models. J Biochem Mol Toxicol 34 https://doi.org/10.1002/jbt.22429
Muhammad M, Ayo J, Danjuma N et al (2019) Molecular mechanisms of aluminium neurotoxicity in animal models of Alzheimer’s disease. J African Assoc Physiol Sci 7:70–79
Sun W, Li J, Li X et al (2022) Aluminium oxide nanoparticles compromise spatial memory performance and proBDNF-mediated neuronal function in the hippocampus of rats. Part Fibre Toxicol 19:34. https://doi.org/10.1186/s12989-022-00477-8
Niño SA, Morales-Martínez A, Chi-Ahumada E et al (2019) Arsenic exposure contributes to the bioenergetic damage in an Alzheimer’s disease model. ACS Chem Neurosci 10:323–336. https://doi.org/10.1021/acschemneuro.8b00278
Karim Y, Siddique AE, Hossen F et al (2019) Dose-dependent relationships between chronic arsenic exposure and cognitive impairment and serum brain-derived neurotrophic factor. Environ Int 131:105029. https://doi.org/10.1016/J.ENVINT.2019.105029
Du X, Tian M, Wang X et al (2018) Cortex and hippocampus DNA epigenetic response to a long-term arsenic exposure via drinking water. Environ Pollut 234:590–600. https://doi.org/10.1016/j.envpol.2017.11.083
Bjørklund G, Tinkov AA, Dadar M et al (2019) Insights into the potential role of mercury in Alzheimer’s disease. J Mol Neurosci 67:511–533. https://doi.org/10.1007/s12031-019-01274-3
Meleleo D, Notarachille G, Mangini V, Arnesano F (2019) Concentration-dependent effects of mercury and lead on Aβ42: possible implications for Alzheimer’s disease. Eur Biophys J 48:173–187. https://doi.org/10.1007/s00249-018-1344-9
Wallin C, Friedemann M, Sholts SB et al (2019) Mercury and Alzheimer’s disease: Hg(II) ions display specific binding to the amyloid-β peptide and hinder its fibrillization. Biomolecules 10:44. https://doi.org/10.3390/biom10010044
Siblerud R, Mutter J, Moore E et al (2019) A hypothesis and evidence that mercury may be an etiological factor in Alzheimer’s disease. Int J Environ Res Public Health 16:5152. https://doi.org/10.3390/ijerph16245152
Liu R, Bai L, Liu M et al (2022) Combined exposure of lead and high-fat diet enhanced cognitive decline via interacting with CREB-BDNF signaling in male rats. Environ Pollut 304:119200. https://doi.org/10.1016/J.ENVPOL.2022.119200
Eleiwa NZH, Ali MAA, Said EN et al (2023) Bee venom (Apis mellifera L.) rescues zinc oxide nanoparticles induced neurobehavioral and neurotoxic impact via controlling neurofilament and GAP-43 in rat brain. Environ Sci Pollut Res 1:1–19. https://doi.org/10.1007/S11356-023-28538-1
Johansson N, Eriksson P, Viberg H (2009) Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci 108:412–418. https://doi.org/10.1093/TOXSCI/KFP029
Kobayashi NR, Fan DP, Giehl KM et al (1997) BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci 17:9583–9595. https://doi.org/10.1523/JNEUROSCI.17-24-09583.1997
Lee YJ, Jeong YJ, Kang EJ et al (2023) GAP-43 closely interacts with BDNF in hippocampal neurons and is associated with Alzheimer’s disease progression. Front Mol Neurosci 16:1150399. https://doi.org/10.3389/FNMOL.2023.1150399/BIBTEX
Syeda T, Cannon JR (2021) Environmental exposures and the etiopathogenesis of Alzheimer’s disease: the potential role of BACE1 as a critical neurotoxic target. J Biochem Mol Toxicol 35:e22694. https://doi.org/10.1002/JBT.22694
Lin R, Chen X, Li W et al (2008) Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neurosci Lett 440:344–347. https://doi.org/10.1016/J.NEULET.2008.05.070
Wang R, Wu Z, Liu R et al (2022) Age-related miRNAs dysregulation and abnormal BACE1 expression following Pb exposure in adolescent mice. Environ Toxicol 37:1902–1913. https://doi.org/10.1002/TOX.23536
Yan W, Yun Y, Ku T et al (2016) NO2 inhalation promotes Alzheimer’s disease-like progression: cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci Rep 6:1–17. https://doi.org/10.1038/srep22429
Yasmin Nisa F, Atiar Rahman M, Amjad Hossen M et al (2021) Role of neurotoxicants in the pathogenesis of Alzheimer’s disease: a mechanistic insight. Ann Med 53:1479–1504. https://doi.org/10.1080/07853890.2021.1966088
Gassowska M, Baranowska-Bosiacka I, Moczydłowska J et al (2016) Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3β and CDK5 dependent manner: relevance to neurological disorders. Toxicology 347–349:17–28. https://doi.org/10.1016/J.TOX.2016.03.002
Singh SA, Suresh S, Singh A et al (2022) Perspectives of ozone induced neuropathology and memory decline in Alzheimer’s disease: a systematic review of preclinical evidences. Environ Pollut 313:120136. https://doi.org/10.1016/J.ENVPOL.2022.120136
Abeti R, Duchen MR (2012) Activation of PARP by oxidative stress induced by β-amyloid: implications for Alzheimer’s disease. Neurochem Res 37:2589–2596. https://doi.org/10.1007/S11064-012-0895-X/FIGURES/1
Pascal JM (2018) The comings and goings of PARP-1 in response to DNA damage. DNA Repair (Amst) 71:177–182. https://doi.org/10.1016/J.DNAREP.2018.08.022
Salech F, Ponce DP, Paula-Lima AC et al (2020) Nicotinamide, a poly [ADP-ribose] polymerase 1 (PARP-1) inhibitor, as an adjunctive therapy for the treatment of Alzheimer’s disease. Front Aging Neurosci 12:255. https://doi.org/10.3389/FNAGI.2020.00255/BIBTEX
Chuang Y, Van I, Zhao Y, Xu Y (2021) Icariin ameliorate Alzheimer’s disease by influencing SIRT1 and inhibiting Aβ cascade pathogenesis. J Chem Neuroanat 117:102014. https://doi.org/10.1016/J.JCHEMNEU.2021.102014
Yin Z, Gao D, Du K, et al. (2022) Rhein ameliorates cognitive impairment in an APP/PS1 transgenic mouse model of Alzheimer’s disease by relieving oxidative stress through activating the SIRT1/PGC-1 α pathway. Oxid Med Cell Longev 2022https://doi.org/10.1155/2022/2524832
Liang Q, Zhang Y, Huang M et al (2019) Role of mitochondrial damage in Cr(VI)-induced endoplasmic reticulum stress in L-02 hepatocytes. Mol Med Rep 19:1256–1265. https://doi.org/10.3892/MMR.2018.9704/HTML
Li H, Shi J, Gao H et al (2022) Hexavalent chromium causes apoptosis and autophagy by inducing mitochondrial dysfunction and oxidative stress in broiler cardiomyocytes. Biol Trace Elem Res 200:2866–2875. https://doi.org/10.1007/S12011-021-02877-X/FIGURES/6
Liu H, Han W, Zhu S et al (2021) Effect of DEHP and DnOP on mitochondrial damage and related pathways of Nrf2 and SIRT1/PGC-1α in HepG2 cells. Food Chem Toxicol 158:112696. https://doi.org/10.1016/J.FCT.2021.112696
Ijomone OM, Iroegbu JD, Aschner M, Bornhorst J (2021) Impact of environmental toxicants on p38- and ERK-MAPK signaling pathways in the central nervous system. Neurotoxicology 86:166–171. https://doi.org/10.1016/J.NEURO.2021.08.005
Phuagkhaopong S, Ospondpant D, Kasemsuk T et al (2017) Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology 60:82–91. https://doi.org/10.1016/J.NEURO.2017.03.001
Tai SH, Huang SY, Chao LC et al (2022) Lithium upregulates growth-associated protein-43 (GAP-43) and postsynaptic density-95 (PSD-95) in cultured neurons exposed to oxygen-glucose deprivation and improves electrophysiological outcomes in rats subjected to transient focal cerebral ischemia foll. Neurol reseaarchrch 44:870–878. https://doi.org/10.1080/01616412.2022.2056817
Cascella M, Bimonte S, Muzio MR, et al. (2017) The efficacy of epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practice 1–7 https://doi.org/10.1186/s13027-017-0145-6
Valverde-Salazar V, Ruiz-Gabarre D, García-Escudero V (2023) Alzheimer’s disease and green tea: epigallocatechin-3-gallate as a modulator of inflammation and oxidative stress. Antioxidants 12:1460. https://doi.org/10.3390/ANTIOX12071460
Lagraoui M, Sukumar G, Latoche JR et al (2017) Salsalate treatment following traumatic brain injury reduces inflammation and promotes a neuroprotective and neurogenic transcriptional response with concomitant functional recovery. Brain Behav Immun 61:96–109. https://doi.org/10.1016/J.BBI.2016.12.005
Pasinetti GM, Wang J, Ho L et al (2015) Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta - Mol Basis Dis 1852:1202–1208. https://doi.org/10.1016/J.BBADIS.2014.10.006
Kihara T, Shimmyo Y, Akaike A et al (2010) Abeta-induced BACE-1 cleaves N-terminal sequence of mPGES-2. Biochem Biophys Res Commun 393:728–733. https://doi.org/10.1016/J.BBRC.2010.02.069
Alam JJ (2015) Selective brain-targeted antagonism of p38 MAPKα reduces hippocampal IL-1β levels and improves Morris water maze performance in aged rats. J Alzheimer’s Dis 48:219–227. https://doi.org/10.3233/JAD-150277
Dong Y, Li X, Cheng J, Hou L (2019) Drug development for alzheimer’s disease: microglia induced neuroinflammation as a target? Int J Mol Sci 20 https://doi.org/10.3390/IJMS20030558
Chen Y, Li L, Zhang J et al (2021) Dexmedetomidine alleviates lipopolysaccharide-induced hippocampal neuronal apoptosis via inhibiting the p38 MAPK/c-Myc/CLIC4 signaling pathway in rats. Mol Neurobiol 58:5533–5547. https://doi.org/10.1007/S12035-021-02512-9/FIGURES/8
Guo J-W, Guan P-P, Ding W-Y et al (2017) Erythrocyte membrane-encapsulated celecoxib improves the cognitive decline of Alzheimer’s disease by concurrently inducing neurogenesis and reducing apoptosis in APP/PS1 transgenic mice. Biomaterials 145:106–127. https://doi.org/10.1016/j.biomaterials.2017.07.023
Mhillaj E, Morgese MG, Tucci P et al (2018) Celecoxib prevents cognitive impairment and neuroinflammation in soluble amyloid β-treated rats. Neuroscience 372:58–73. https://doi.org/10.1016/J.NEUROSCIENCE.2017.12.046
Morales-Garcia JA, Luna-Medina R, Alonso-Gil S et al (2012) Glycogen synthase kinase3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci 3:963. https://doi.org/10.1021/CN300110C
Athar T, Al Balushi K, Khan SA (2021) Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep 48:5629–5645. https://doi.org/10.1007/S11033-021-06512-9/METRICS
Koriyama Y, Hori A, Ito H et al (2021) Discovery of atabecestat (JNJ-54861911): a thiazine-based β-amyloid precursor protein cleaving enzyme 1 inhibitor advanced to the phase 2b/3 early clinical trial. J Med Chem 64:1873–1888. https://doi.org/10.1021/ACS.JMEDCHEM.0C01917/SUPPL_FILE/JM0C01917_SI_002.CSV
Zakaria JAD, Vassar RJ (2018) A promising, novel, and unique BACE1 inhibitor emerges in the quest to prevent Alzheimer’s disease. EMBO Mol Med 10:e9717. https://doi.org/10.15252/EMMM.201809717
Novak G, Streffer JR, Timmers M et al (2020) Long-term safety and tolerability of atabecestat (JNJ-54861911), an oral BACE1 inhibitor, in early Alzheimer’s disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimer’s Res Ther 12:1–16. https://doi.org/10.1186/S13195-020-00614-5/TABLES/6
(2023) Lead (Pb) toxicity: what are U.S. standards for lead levels? | Environmental Medicine. In: Agency toxic Subst. Dis. Regist. https://www.atsdr.cdc.gov/csem/leadtoxicity/safety_standards.html. Accessed 9 Aug 2023
Brown EE, Gerretsen P, Pollock B, Graff-Guerrero A (2018) Psychiatric benefits of lithium in water supplies may be due to protection from the neurotoxicity of lead exposure. Med Hypotheses 115:94–102. https://doi.org/10.1016/J.MEHY.2018.04.005
Wang X, An Y, Jiao W et al (2018) Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys. Biol Trace Elem Res 182:354–363. https://doi.org/10.1007/S12011-017-1097-9/FIGURES/4
Bihaqi SW (2019) Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease. Rev Environ Health 34:187–195. https://doi.org/10.1515/reveh-2018-0076
Bandaru LJM, Ayyalasomayajula N, Murumulla L, Challa S (2022) Mechanisms associated with the dysregulation of mitochondrial function due to lead exposure and possible implications on the development of Alzheimer’s disease. Biometals 35:1–25. https://doi.org/10.1007/s10534-021-00360-7
Kobza J, Geremek M, Dul L (2021) Ozone concentration levels in urban environments—Upper Silesia region case study. Int J Environ Res Public Health 18:1–20. https://doi.org/10.3390/IJERPH18041473
(2023) National Ambient Air Quality Standards (NAAQS) for PM. In: US EPA. https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm. Accessed 9 Aug 2023
Thangavel P, Park D, Lee Y-C (2022) Recent insights into particulate matter (PM(2.5))-mediated toxicity in humans: an overview. Int J Environ Res Public Health 19 https://doi.org/10.3390/ijerph19127511
(2022) Copper — health professional fact sheet. In: Natl. Inst. Heal. https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/. Accessed 9 Aug 2023
Linder MC (2012) The relationship of copper to DNA damage and damage prevention in humans. Mutat Res 733:83–91. https://doi.org/10.1016/j.mrfmmm.2012.03.010
Bayoumi AE, Bayoumi AE (2022) Deleterious effects of banned chemical pesticides on human health in developing countries. In: Pesticides - Updates on Toxicity, Efficacy and Risk Assessment. IntechOpen, pp169–194
(2023) Cadmium toxicity: where is cadmium found? | Environmental Medicine. In: Agency toxic Subst. Dis. Regist. https://www.atsdr.cdc.gov/csem/cadmium/where-cadmium-found.html. Accessed 9 Aug 2023
Branca JJV, Morucci G, Becatti M et al (2019) Cannabidiol protects dopaminergic neuronal cells from cadmium. Int J Environ Res Public Heal 16:4420. https://doi.org/10.3390/IJERPH16224420
Naija A, Yalcin HC (2023) Evaluation of cadmium and mercury on cardiovascular and neurological systems: effects on humans and fish. Toxicol Rep 10:498–508. https://doi.org/10.1016/J.TOXREP.2023.04.009
Rahimzadeh MR, Rahimzadeh MR, Kazemi S et al (2022) Aluminum poisoning with emphasis on its mechanism and treatment of intoxication. Emerg Med Int 2022:1–13. https://doi.org/10.1155/2022/1480553
Shrivastava BK, Vani A (2009) Comparative study of defluoridation technologies in India. Asian J Exp Sci 23:269–274
Campbell A (2002) The potential role of aluminium in Alzheimer’s disease. Nephrol Dial Transplant 17(Suppl 2):17–20. https://doi.org/10.1093/NDT/17.SUPPL_2.17
Touqeer A (2021) Biochemical mechanisms of aluminium induced neurological disorders. Bentham Sci. https://doi.org/10.2174/97816810888391210101
(2023) Arsenic toxicity: what are the standards and regulation for arsenic exposure? | Environmental Medicine. In: Agency toxic Subst. Dis. Regist. https://www.atsdr.cdc.gov/csem/arsenic/standards.html. Accessed 9 Aug 2023
Rahman MA, Hannan MA, Uddin MJ, et al. (2021) Exposure to environmental arsenic and emerging risk of Alzheimer’s disease: perspective mechanisms, management strategy, and future directions. Toxics 9 https://doi.org/10.3390/TOXICS9080188
Muzaffar S, Khan J, Srivastava R et al (2023) Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol Toxicol 39:85–110. https://doi.org/10.1007/s10565-022-09710-8
Azar J, Yousef MH, El-Fawal HAN, Abdelnaser A (2021) Mercury and Alzheimer’s disease: a look at the links and evidence. Metab Brain Dis 36:361–374. https://doi.org/10.1007/S11011-020-00649-5/FIGURES/4
Alemany S, Crous-Bou M, Vilor-Tejedor N et al (2021) Associations between air pollution and biomarkers of Alzheimer’s disease in cognitively unimpaired individuals. Environ Int 157:106864. https://doi.org/10.1016/J.ENVINT.2021.106864
Falcón C, Gascon M, Molinuevo JL et al (2021) Brain correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk for Alzheimer’s disease: a study on Barcelona’s population. Alzheimer’s Dement Diagnosis Assess Dis Monit 13:e12205. https://doi.org/10.1002/DAD2.12205
Campbell A, Oldham M, Becaria A et al (2005) Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26:133–140. https://doi.org/10.1016/J.NEURO.2004.08.003
Bihaqi SW, Eid A, Zawia NH (2017) Lead exposure and tau hyperphosphorylation: an in vitro study. Neurotoxicology 62:218–223. https://doi.org/10.1016/J.NEURO.2017.07.029
Zhang A, Matsushita M, Zhang L et al (2021) Cadmium exposure modulates the gut-liver axis in an Alzheimer’s disease mouse model. Commun Biol 4:1–16. https://doi.org/10.1038/s42003-021-02898-1
Kinawy AA (2019) Effects of drinking water containing aluminum and fluoride salts on the learning behavior and brain neurotransmitters of male rat offspring. Egypt J Zool 71:1–12. https://doi.org/10.12816/ejz.2019.9821.1006
Chabuk HA, Al-Harbi HJ, Al-Saadi HKZ (2019) Aluminum chloride administration induced behavioral and physiological changes in adult male rats. Indian J Public Heal Res Dev 10:3721–3725
Hadjer B, Menadi Noureddine DA, Hichem M et al (2019) Neurotoxicity of heavy metals (aluminum chloride) studies performed on rats wistar. Int J Biosci 15:431–437. https://doi.org/10.12692/ijb/15.1.431-437
Wisessaowapak C, Visitnonthachai D, Watcharasit P, Satayavivad J (2021) Prolonged arsenic exposure increases tau phosphorylation in differentiated SH-SY5Y cells: the contribution of GSK3 and ERK1/2. Environ Toxicol Pharmacol 84:103626. https://doi.org/10.1016/J.ETAP.2021.103626
Shaw P, Mondal P, Bandyopadhyay A, Chattopadhyay A (2020) Environmentally relevant concentration of chromium induces nuclear deformities in erythrocytes and alters the expression of stress-responsive and apoptotic genes in brain of adult zebrafish. Sci Total Environ 703:135622. https://doi.org/10.1016/J.SCITOTENV.2019.135622
Xu Y, Wang X, Geng N et al (2020) Mitophagy is involved in chromium (VI)-induced mitochondria damage in DF-1 cells. Ecotoxicol Environ Saf 194:110414. https://doi.org/10.1016/J.ECOENV.2020.110414
Lange KW, Lange KM, Nakamura Y (2022) Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence. Food Sci Hum Wellness 11:765–770. https://doi.org/10.1016/j.fshw.2022.03.002
Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S et al (2007) Neurobiology of disease NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. Neurobiol Dis 27:5766–5776. https://doi.org/10.1523/JNEUROSCI.1004-07.2007
Funding
D. H. K. R. received research support from the UGC-BSR (No. F.30–583/2021(BSR) and Central University of Punjab, Bathinda—Research Seed Money (CUPB/CC/PF/20/226). R. D. is the recipient of research fellowship from the Department of Science and Technology—DST-INSPIRE (Reg. No. IF210098), P. S. is the recipient of non-NET fellowship (Ref. No. CUPB/Acad.-54/2022–23/Notification/2472) from Central University of Punjab, and S. K. is the recipient of fellowship AICTE.
Author information
Authors and Affiliations
Contributions
D. H. K. R. designed the manuscript. R. D., P. S., and S. K. drafted the manuscript. D. H. R. and J. S. B. revised the manuscript for important intellectual content. R. D. and P. S. prepared the illustrated figures. R. D. and S. K. prepared the tables. All authors read and approved the final manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Ethics Approval
Yes.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhapola, R., Sharma, P., Kumari, S. et al. Environmental Toxins and Alzheimer’s Disease: a Comprehensive Analysis of Pathogenic Mechanisms and Therapeutic Modulation. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03805-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03805-x