Abstract
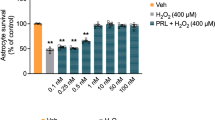
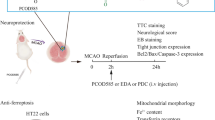
The role of hydrogen sulfide (H2S) on the phenotypic change of astrocytes following cerebral ischemia/reperfusion (I/R) in mice was investigated in present study. We tested the expression of glial fibrillary acidic protein (GFAP), A2 phenotype marker S100a10, and A1 phenotype marker C3 protein and assessed the change of BrdU/GFAP-positive cells, GFAP/C3-positive cells, and GFAP/S100a10-positive cells in mice hippocampal tissues to evaluate the change of astrocyte phenotypes following cerebral I/R. The role of H2S on the phenotypic change of astrocytes following cerebral I/R in mice was investigated by using H2S synthase cystathionine-γ-lyase (CSE) knockout mice (KO). The results revealed that cerebral I/R injury promoted the astrocytes proliferation of both A1 and A2 phenotypes, which were more significant in mice of H2S synthase CSE KO than in mice of wild type (WT). Interestingly, supplement with H2S could inhibit the A1 phenotype proliferation but promote the proliferation of A2 phenotype, suggesting that H2S could regulate the transformation of astrocytes to A2 phenotype following cerebral I/R, which is beneficial for neuronal recovery. Besides, we found that H2S-mediated change of astrocyte phenotype is related to inhibiting the RhoA/ROCK pathway. Furthermore, both H2S and ROCK inhibitor could ameliorate the brain injury of mice at 9 days after cerebral I/R. In conclusion, H2S regulates the phenotypic transformation of astrocytes to A2 phenotype following the cerebral I/R via inhibiting RhoA/ROCK pathway and then exerts the neuroprotective effect against the subacute brain injury.











Similar content being viewed by others
Data Availability
The data and materials are available on reasonable request.
Abbreviations
- WT:
-
Wild type
- CSE KO:
-
CSE knockout
- CCAs:
-
Bilateral common carotid arteries
- GFAP:
-
Glial fibrillary acidic protein
- ROCK:
-
Rho kinase
- CNS:
-
Cerebral nervous system
- H2S:
-
Hydrogen sulfide
- CO:
-
Monoxide
- NO:
-
Nitric oxide
- CSE:
-
Cystathionine γ-lyase
- CBS:
-
Cystathionine β-synthase
- 3-MST:
-
3-Mercaptopyruvate sulfurtransferase
- BrdU:
-
Bromodeoxyuridine
- OFT:
-
Open-field test
- MWM:
-
Morris water maze
- H&E:
-
Hematoxylin and eosin staining
- NSE:
-
Neuron-specific enolase
- LDH:
-
Lactate dehydrogenase
- PAGE:
-
SDS-polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene difluoride
- DAPI:
-
Anti-4′,6-diamidino-2-phenylindole
- MBP:
-
Myelin basic protein
- NeuN:
-
Neuronal nuclei
References
Herpich F, Rincon F (2020) Management of acute ischemic stroke. Crit Care Med 48(11):1654–1663. https://doi.org/10.1097/CCM.0000000000004597
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A (2004) Targets for vascular protection after acute ischemic stroke. Stroke 35(9):2220–2225. https://doi.org/10.1161/01.STR.0000138023.60272.9e
Paul S, Candelario-Jalil E (2021) Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 335:113518. https://doi.org/10.1016/j.expneurol.2020.113518
Wu D, Wang J, Li H, Xue M, Ji A, Li Y (2015) Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid Med Cell Longev 2015:186908. https://doi.org/10.1155/2015/186908
Deng G, Muqadas M, Adlat S, Zheng H, Li G, Zhu P, Nasser MI (2023) Protective effect of hydrogen sulfide on cerebral ischemia-reperfusion injury. Cell Mol Neurobiol 43(1):15–25. https://doi.org/10.1007/s10571-021-01166-4
Powell CR, Dillon KM, Matson JB (2018) A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol 149:110–123. https://doi.org/10.1016/j.bcp.2017.11.014
Wen JY, Wang M, Li YN, Jiang HH, Sun XJ, Chen ZW (2018) Vascular protection of hydrogen sulfide on cerebral ischemia/reperfusion injury in rats. Front Neurol 9:779. https://doi.org/10.3389/fneur.2018.00779
Wen JY, Gao SS, Chen FL, Chen S, Wang M, Chen ZW (2019) Role of CSE-produced H2S on cerebrovascular relaxation via RhoA-ROCK inhibition and cerebral ischemia-reperfusion injury in mice. ACS Chem Neurosci 10(3):1565–1574. https://doi.org/10.1021/acschemneuro.8b00533
Zhang Y, Li K, Wang X, Ding Y, Ren Z, Fang J, Sun T, Guo Y et al (2021) CSE-derived H(2)S inhibits reactive astrocytes proliferation and promotes neural functional recovery after cerebral ischemia/reperfusion injury in mice via inhibition of RhoA/ROCK(2) pathway. ACS Chem Neurosci 12(14):2580–2590. https://doi.org/10.1021/acschemneuro.0c00674
Escartin C, Galea E, Lakatos A, O’callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Guo H, Fan Z, Wang S, Ma L, Wang J, Yu D, Zhang Z, Wu L et al (2021) Astrocytic A1/A2 paradigm participates in glycogen mobilization mediated neuroprotection on reperfusion injury after ischemic stroke. J Neuroinflammation 18(1):230. https://doi.org/10.1186/s12974-021-02284-y
Chen J, Yin W, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y et al (2017) L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: Involvement of NF-kappaB inhibition and Nrf2 pathway activation. Eur J Pharmacol 806:1–9. https://doi.org/10.1016/j.ejphar.2017.03.025
Han Y, Li X, Yang L, Zhang D, Li L, Dong X, Li Y, Qun S et al (2022) Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J Ginseng Res 46(4):515–525. https://doi.org/10.1016/j.jgr.2021.08.001
Ding Y, Liu B, Zhang Y, Fang F, Li X, Wang S, Wen J (2022) Hydrogen sulphide protects mice against the mutual aggravation of cerebral ischaemia/reperfusion injury and colitis. Eur J Pharmacol 914:174682. https://doi.org/10.1016/j.ejphar.2021.174682
Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, Xu LZ, Wu WN (2020) NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation 17(1):178. https://doi.org/10.1186/s12974-020-01848-8
Zhang Y, Li K, Wang X, Ding Y, Ren Z, Fang J, Sun T, Guo Y et al (2021) CSE-derived H2S inhibits reactive astrocytes proliferation and promotes neural functional recovery after cerebral ischemia/reperfusion injury in mice via inhibition of RhoA/ROCK2 pathway. ACS Chem Neurosci 12(14):2580–2590. https://doi.org/10.1021/acschemneuro.0c00674
Sun YW, Luo YP, Zheng XL, Wu XY, Wen HZ, Hou WS (2021) Multiple sessions of entorhinal cortex deep brain stimulation in C57BL/6J mice increases exploratory behavior and hippocampal neurogenesis(). Annu Int Conf IEEE Eng Med Biol Soc 2021:6390–6393. https://doi.org/10.1109/EMBC46164.2021.9629978
Chen Y, Wen J, Chen Z (2021) H2S protects hippocampal neurons against hypoxia-reoxygenation injury by promoting RhoA phosphorylation at Ser188. Cell Death Discov 7(1):132. https://doi.org/10.1038/s41420-021-00514-z
Wen JY, Zhang J, Chen S, Chen Y, Zhang Y, Ma ZY, Zhang F, Xie WM et al (2021) Endothelium-derived hydrogen sulfide acts as a hyperpolarizing factor and exerts neuroprotective effects via activation of large-conductance Ca(2+) -activated K(+) channels. Br J Pharmacol 178(20):4155–4175. https://doi.org/10.1111/bph.15607
Cheng X, Yeung PKK, Zhong K, Zilundu PLM, Zhou L, Chung SK (2019) Astrocytic endothelin-1 overexpression promotes neural progenitor cells proliferation and differentiation into astrocytes via the Jak2/Stat3 pathway after stroke. J Neuroinflammation 16(1):227. https://doi.org/10.1186/s12974-019-1597-y
Miyamoto N, Magami S, Inaba T, Ueno Y, Hira K, Kijima C, Nakajima S, Yamashiro K et al (2020) The effects of A1/A2 astrocytes on oligodendrocyte linage cells against white matter injury under prolonged cerebral hypoperfusion. Glia 68(9):1910–1924. https://doi.org/10.1002/glia.23814
Hernandez IH, Villa-Gonzalez M, Martin G, Soto M, Perez-Alvarez MJ (2021) Glial cells as therapeutic approaches in brain ischemia-reperfusion injury. Cells 10(7):1639. https://doi.org/10.3390/cells10071639
Liu Y, Meng X, Sun L, Pei K, Chen L, Zhang S, Hu M (2022) Protective effects of hydroxy-alpha-sanshool from the pericarp of Zanthoxylum bungeanum Maxim. On D-galactose/AlCl3-induced Alzheimer’s disease-like mice via Nrf2/HO-1 signaling pathways. Eur J Pharmacol 914:174691. https://doi.org/10.1016/j.ejphar.2021.174691
Guo S, Wang R, Hu J, Sun L, Zhao X, Zhao Y, Han D, Hu S (2021) Photobiomodulation promotes hippocampal CA1 NSC differentiation toward neurons and facilitates cognitive function recovery involving NLRP3 inflammasome mitigation following global cerebral ischemia. Front Cell Neurosci 15:731855. https://doi.org/10.3389/fncel.2021.731855
Zheng J, Zhang T, Han S, Liu C, Liu M, Li S, Li J (2021) Activin A improves the neurological outcome after ischemic stroke in mice by promoting oligodendroglial ACVR1B-mediated white matter remyelination. Exp Neurol 337:113574. https://doi.org/10.1016/j.expneurol.2020.113574
Li Y, Yao N, Zhang T, Guo F, Niu X, Wu Z, Hou S (2020) Ability of post-treatment glycyrrhizic acid to mitigate cerebral ischemia/reperfusion injury in diabetic mice. Medical Science Monitor 26:926551. https://doi.org/10.12659/MSM.926551
Renner M, Stute G, Alzureiqi M, Reinhard J, Wiemann S, Schmid H, Faissner A, Dick HB et al (2017) Optic nerve degeneration after retinal ischemia/reperfusion in a rodent model. Front Cell Neurosci 11:254. https://doi.org/10.3389/fncel.2017.00254
Kanavaki A, Spengos K, Moraki M, Delaporta P, Kariyannis C, Papassotiriou I, Kattamis A (2017) Serum levels of S100b and NSE proteins in patients with non-transfusion-dependent thalassemia as biomarkers of brain ischemia and cerebral vasculopathy. Int J Mol Sci 18(12):2724. https://doi.org/10.3390/ijms18122724
Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR, Group NR-PSS (2006) Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator stroke study. Stroke 37(10):2508–2513. https://doi.org/10.1161/01.STR.0000242290.01174.9e
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. https://doi.org/10.1007/s00401-009-0619-8
Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S et al (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 23(7):818–828. https://doi.org/10.1038/nm.4354
Shen XY, Gao ZK, Han Y, Yuan M, Guo YS, Bi X (2021) Activation and role of astrocytes in ischemic stroke. Front Cell Neurosci 15:755955. https://doi.org/10.3389/fncel.2021.755955
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504(7480):394–400. https://doi.org/10.1038/nature12776
Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, Nabekura J, Sato K et al (2017) Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun 8(1):28. https://doi.org/10.1038/s41467-017-00037-1
Juric M, Balog M, Ivic V, Benzon B, Racetin A, Bocina I, Kevic N, Konjevoda S et al (2021) Chronic stress and gonadectomy affect the expression of Cx37, Cx40 and Cx43 in the spinal cord. Life (Basel) 11(12):1330. https://doi.org/10.3390/life11121330
Li G, Zhang S, Cheng Y, Lu Y, Jia Z, Yang X, Zhang S, Guo W et al (2022) Baicalin suppresses neuron autophagy and apoptosis by regulating astrocyte polarization in pentylenetetrazol-induced epileptic rats and PC12 cells. Brain Res 1774:147723. https://doi.org/10.1016/j.brainres.2021.147723
Jiang S, Wang H, Zhou Q, Li Q, Liu N, Li Z, Chen C, Deng Y (2021) Melatonin ameliorates axonal hypomyelination of periventricular white matter by transforming A1 to A2 astrocyte via JAK2/STAT3 pathway in septic neonatal rats. J Inflamm Res 14:5919–5937. https://doi.org/10.2147/JIR.S337499
Bros M, Haas K, Moll L, Grabbe S (2019) RhoA as a key regulator of innate and adaptive immunity. Cells 8(7):733. https://doi.org/10.3390/cells8070733
Koch JC, Tonges L, Barski E, Michel U, Bahr M, Lingor P (2014) ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis 5:e1225. https://doi.org/10.1038/cddis.2014.191
Gong P, Li R, Jia HY, Ma Z, Li XY, Dai XR, Luo SY (2020) Anfibatide preserves blood-brain barrier integrity by inhibiting TLR4/RhoA/ROCK pathway after cerebral ischemia/reperfusion injury in rat. J Mol Neurosci 70(1):71–83. https://doi.org/10.1007/s12031-019-01402-z
Zhang Y, Miao L, Peng Q, Fan X, Song W, Yang B, Zhang P, Liu G et al (2022) Parthenolide modulates cerebral ischemia-induced microglial polarization and alleviates neuroinflammatory injury via the RhoA/ROCK pathway. Phytomedicine 105:154373. https://doi.org/10.1016/j.phymed.2022.154373
Chen Y, Wen J, Chen Z (2021) H(2)S protects hippocampal neurons against hypoxia-reoxygenation injury by promoting RhoA phosphorylation at Ser188. Cell Death Discov 7(1):132. https://doi.org/10.1038/s41420-021-00514-z
Fang F, Sheng J, Guo Y, Wen J, Chen Z (2023) Protection of H(2)S against hypoxia/reoxygenation injury in rat hippocampal neurons through inhibiting phosphorylation of ROCK(2) at Thr436 and Ser575. Pharmaceuticals (Basel, Switzerland) 16(2):218. https://doi.org/10.3390/ph16020218
Liu Y, Liao S, Quan H, Lin Y, Li J, Yang Q (2016) Involvement of microRNA-135a-5p in the protective effects of hydrogen sulfide against Parkinson’s disease. Cell Physiol Biochem 40(1–2):18–26. https://doi.org/10.1159/000452521
Chen J, Sun Z, Jin M, Tu Y, Wang S, Yang X, Chen Q, Zhang X et al (2017) Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-kappaB pathway. J Neuroimmunol 305:108–114. https://doi.org/10.1016/j.jneuroim.2017.02.010
Tsukahara R, Ueda H (2016) Myelin-related gene silencing mediated by LPA1 - Rho/ROCK signaling is correlated to acetylation of NFkappaB in S16 Schwann cells. J Pharmacol Sci 132(2):162–165. https://doi.org/10.1016/j.jphs.2016.07.010
Zhu L, Chen T, Chang X, Zhou R, Luo F, Liu J, Zhang K, Wang Y et al (2016) Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-kappaB pathway. Neuropharmacology 103:134–142. https://doi.org/10.1016/j.neuropharm.2015.12.007
Guo H, Yang J, Liu M, Wang L, Hou W, Zhang L, Ma Y (2020) Selective activation of estrogen receptor beta alleviates cerebral ischemia neuroinflammatory injury. Brain Res 1726:146536. https://doi.org/10.1016/j.brainres.2019.146536
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J et al (2015) NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85(1):101–115. https://doi.org/10.1016/j.neuron.2014.11.018
Moreno-Garcia A, Bernal-Chico A, Colomer T, Rodriguez-Antiguedad A, Matute C, Mato S (2020) Gene expression analysis of astrocyte and microglia endocannabinoid signaling during autoimmune demyelination. Biomolecules 10(9):1228. https://doi.org/10.3390/biom10091228
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Zhang Y, Wang X, Jiang C, Chen Z, Ni S, Fan H, Wang Z, Tian F et al (2022) Rho kinase inhibitor Y27632 improves recovery after spinal cord injury by shifting astrocyte phenotype and morphology via the ROCK/NF-kappaB/C3 pathway. Neurochem Res 47(12):3733–3744. https://doi.org/10.1007/s11064-022-03756-0
Funding
This study was supported by Natural Science Foundation of Colleges and Universities in Anhui Province (No. 2023AH050672) and Natural Science Foundation of Anhui Province (NO. 2308085MH302).
Author information
Authors and Affiliations
Contributions
JW and ZC designed the manuscript. YD, XL, and FF carried out experiments and wrote the manuscript. XL and XY performed the behavioral experiments in mice. YD performed the ELISA and analyzed the data. SS, JW, and ZC contributed to funding acquisition, supervision, and editing.
Corresponding authors
Ethics declarations
Ethics Approval
The animal experiments were approved by the Ethical Committee of Anhui Medical University, and all experimental protocols complied with the regulations set by animal care and use committee in Anhui Medical University, which comply with the protocol outlined in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 2011).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, Y., Fang, F., Liu, X. et al. H2S Regulates the Phenotypic Transformation of Astrocytes Following Cerebral Ischemia/Reperfusion via Inhibiting the RhoA/ROCK Pathway. Mol Neurobiol 61, 3179–3197 (2024). https://doi.org/10.1007/s12035-023-03797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03797-8