Abstract
AFG3L2 is a zinc metalloprotease and an ATPase localized in an inner mitochondrial membrane involved in mitochondrial quality control of several nuclear- and mitochondrial-encoded proteins. Mutations in AFG3L2 lead to diseases like slow progressive ataxia, which is a neurological disorder. This review delineates the cellular functions of AFG3L2 and its dysfunction that leads to major clinical outcomes, which include spinocerebellar ataxia type 28, spastic ataxia type 5, and optic atrophy type 12. It summarizes all relevant AFG3L2 mutations associated with the clinical outcomes to understand the detailed mechanisms attributable to its structure-related multifaceted roles in proteostasis and quality control. We face early diagnostic challenges of ataxia and optic neuropathy due to asymptomatic parents and variable clinical manifestations due to heterozygosity/homozygosity of AFG3L2 mutations. This review intends to promote AFG3L2 as a putative prognostic or diagnostic marker.
Graphical Abstract
Functions, mutations, and clinical manifestations in AFG3L2, a mitochondrial AAA + ATPases.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria, as an organelle, plays a vital role in numerous life-sustaining activities in eukaryotic cells, for example, adenosine triphosphate (ATP) synthesis, calcium homeostasis, and beta-oxidation of fatty acids [1, 2]. Regulated proteolysis of mitochondrial proteome is essential for proper mitochondrial function. Alteration in the mitochondrial protein level may adversely impact energy production, calcium signaling, and other activities in eukaryotic cells leading to various diseases such as cardiovascular diseases and late-onset neurodegenerative diseases [3,4,5,6]. Deregulation of mitochondrial proteome is correlated with mitochondrial diseases such as dominant optic neuropathy, Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease, sarcopenia, and bipolar disorder [7,8,9]. Several proteases play an important role in maintaining mitochondrial proteostasis. One example of a protease that is involved in mitochondrial proteostasis is AFG3L2. AFG3L2 is a mitochondrial ATPase associated with diverse cellular activities (AAA) protease, which is found in the inner membrane of mitochondria [10]. It can function solely as a homo-hexamer or in combination with a protease, paraplegin (encoded by SPG7), in the form of a hetero-hexamer [11]. AFG3L2 has a varied role in cellular physiology. It is involved in the axonal anterograde transport of mitochondria [8], mitochondrial protein synthesis, and cellular respiration [12]. AFG3L2 mutations cause three monogenic disorders which are spinocerebellar ataxia type 28 (SCA28), spastic ataxia type 5 (SPAX5), and optic atrophy type 12 (OPA12) and have been reported for their mechanistic association with other diseases such as PD and other eye associated ataxias like ophthalmoparesis and oculomotor apraxia [9]. It is involved in steroid synthesis regulation via StAR overload response (SOR) [13,14,15,16,17,18,19,20,21,22,23]. This review discusses the multifaceted roles of AFG3L2 in mitochondrial physiology and its role primarily in spinocerebellar ataxia type 28 (SCA28) and other diseases.
AFG3L2: an Inner Mitochondrial Membrane ATPase
AFG3L2 is an inner mitochondrial membrane (IMM) ATPase and a zinc metalloprotease. YME1L, OMA1, and paraplegin are a few other examples of mitochondrial ATPases. These proteases are located in the inner mitochondrial membrane. In brief, OMA1, YME1L, AFG3L2, and paraplegin are the mitochondrial AAAs that play a vital role in mitochondrial proteostasis in their active oligomeric forms. AAA proteases located in the IMM are categorized into ATP-dependent and ATP-independent proteases. These proteases, including AFG3L2, are ubiquitously expressed [11]. They are the descendants of highly conserved bacterial FtsH AAA zinc metalloproteases and are sub-grouped as ring-shaped P-loop NTPases [11]. They are also called as the M41 family of zinc metalloproteases [20].
Location and Structure
AFG3L2 is located in the matrix side of IMM (m-AAA) [11]. Other mitochondrial AAAs that play a vital role in mitochondrial proteostasis in their active oligomeric forms are YME1L and OMA1 [11]. They are located in the IMM side of mitochondria (i-AAA). YME1L (Yme1p in yeast) is an ATP-dependent protease while the OMA1 is an ATP-independent protease and both function in their homo-hexameric form [21].
AFG3L2 is an m-AAA that co-localizes and interacts with another m-AAA, paraplegin. To maintain mitochondrial proteostasis, AFG3L2 can function in both homo-hexamer and hetero-hexameric forms with itself or paraplegin [11]. The human gene encoding AFG3L2 was mapped at chromosome 18p11 by Brunella Franco’s laboratory. A mutant variant of AFG3L2 that causes SCA28 was mapped in the SCA locus on chromosome #18 at 18p11.22-q11.2 by Alfredo Brusco’s laboratory in 2006 [24,25,26]. Nuclear encoded Afg3l2, on translocation to the mitochondria, undergoes processing by MPP peptidase followed by further autocatalysis for its maturation [27]. Unlike paraplegin, no isoform of AFG3L2 has been identified till date that is localized outside mitochondria, like in ER, suggesting that hetero-oligomers can be formed only in mitochondria [28]. Hexamers are solely stabilized by ATP binding [29]. Although there is variability in the assembly of murine m-AAA proteases that is largely dependent on the availability of the individual subunits, the hetero-hexamer form of Afg3l2 and paraplegin is prevalent in neuronal cells [30]. Kress and Weber-Ban have summarized the works of Augustin and co-workers that clearly depict the formation of these hexamers, their molecular determinants, and their roles in substrate dislocation from membranes. The ATP-bound hexamer subunits are considered active states. In the hexamer, three alternating ATP-bound subunits together connect with the substrate. The other three subunits remain in the inactive form, but because of ATP hydrolysis, they become active and take the charge of substrate. It has been reported that each subunit controls the ATPase activity of their neighboring subunits. The alternating hexamer states help in loop movements so that always either two or three subunits have the grip on substrate. Substrates can be detached from one subunit by the allosteric inhibitory effect of others. Subunits of hetero-hexamers of AFG3L2/paraplegin around the ATPase ring manipulate every alternating subunit and generate a firing pattern with two alternate groups. Respiratory impairment offers an in vivo read-out and is an indicator of deregulation of m-AAA protease activity [31, 32] that can be used as a diagnostic approach in mitochondrial diseases.
AAA + domains are highly conserved with either classic cross-hatching clade or HCLR stippling clade [33]. m-AAAs include a least conserved N-terminal distal domain, two transmembrane spans, an AAA + ATPase module with walker A/walker B motif, the second region of homology (SRH) transmembrane spanning domain, and a HExxX or HxxEH or HExGH motif containing a C-terminal metalloprotease or proteolytic domain (Fig. 1) [11, 34]. Walker A motif is an ATP binding GX4GKT/S sequence, and walker B motif is hydrophobic (h), i.e., hDD/E sequence with transmembrane domains [33]. We have studied the conserved regions of AFG3L2 by alignment of amino acids of AFG3L2 protein from different organisms, bacteria to humans (Fig. 2). The highly conserved regions of AFG3L2 among different species are shown in blue (Fig. 2). On top of the amino acid sequences, alpha-helical regions are shown with coil structures, and beta-sheet regions are shown with right-sided arrows (Fig. 2). Glynn’s laboratory generated a reconstituted active AFG3L2 with intact protease activity by displacing the transmembrane domains with a CCHEX sequence stimulating hexamerization of the catalytic domains of the protease [29, 34]. Their work clearly depicts how AFG3L2 can maintain its high specificity and yet differentiate between substrates by identifying degron sequences accessible for peptide bond cleavage. Recently, Puchades and co-workers have elaborated a detailed cryo-EM structure of a truncated construct of AFG3L2 comprising the ATPase and peptidase domains (core of AFG3L2, residues 272–797). For a good understanding of the unique characteristics of the stability and activity of AFG3L2, they also showed detailed structure of disease-specific mutations elucidating the structure–function relation of this m-AAA (discussed later in the AFG3L2 mutation section). NMR structural analysis has revealed an intermembrane space domain of AFG3L2 that is located in the membrane periphery and its furthest region interacts with substrates and prohibitins as and when required [35] aiding in membrane stabilization. The C-terminal protease domain has evolved from the i-AAA YME1L that can only recruit soluble substrates to the m-AAA AFG3L2 where it recruits membrane-bound substrates by charged interactions hence maintaining distinct features for these ATPases. Sequential substrate processing steps include (i) substrate recruitment by N- and C-termini of ATPase domain, (ii) ATP-dependent substrate intercalation by pore loop 1 for translocation, (iii) unfolded substrate transfer by pore loop 2 and central protrusion chamber, and (iv) substrate cleavage at zinc-associated protease active site [36]. Like in other AAA + ATPases, pore-loop 1 forms an aromatic spiral staircase that interacts with the substrate and drives translocation. To adopt a membrane-proximal position, the C-terminus encircles the hexamer conferring a complex stability [27]. The substrate translocation is spatially facilitated by the tightly packed aromatic residues immediately in front of pore-loop 1, which provides a lucid configuration of a central channel. The substrate is further transferred to a central protrusion by pore-loop 2 within the protease domain. A staircase, like spirals in N-termini of ATPase domain, and pore loops 1 and 2 help in the hand-held translocation of substrate from this domain to the catalytic core [27]. These domains work in cooperation to drive nucleotide-driven allosteric changes and pull substrate through the central channel as ATP is hydrolyzed [36].
The genomic orientation and the domain structures of human AFG3L2 protein. The AFG3L2 protein consists of 17 exons and 797 amino acids. The gene position is 18p11.21 and a total of 48 kb. The figure represents various mutations that cause SCA28 (red), SPAX5 (blue), optic atrophy (purple), and SCAR (green)
Alignment and substitutions of amino acids in the highly conserved regions of AFG3L2 protein in different study models from bacteria to humans. The alignment is depicted for regions of secondary structures of Homo sapiens (Hsa, human), Mus musculus (Mmu, mouse), Danio rerio (Dre, zebrafish), Drosophila melanogaster (Drm, fruit fly), Saccharomyces cerevisiae (Sce, yeast), and E. coli (Eco, bacteria). This alignment was done in ESPript 3.0 with PDB secondary structure information for similarity index with percent equivalency at a global score of 0.7. Blue boxes are highly conserved regions with all red-colored amino acids showing conservation across those organisms
Primary Functions
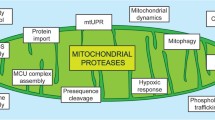
The primary functions of mitochondria include ATP production, fatty acid oxidation, and functioning as calcium ion reservoir [1, 2]. Nuclear-encoded mitochondrial proteins are synthesized in the cytosol and transported into the mitochondria, whereas the mitochondria-encoded proteins are facilitated for co-translational insertion in the inner membrane of mitochondria with simultaneous processing to enable efficient assembly into respective respiratory complexes within the mitochondria [37]. Mitochondrial-encoded proteins being hydrophobic require co-translational insertion into the inner membrane and further require quality control simultaneously for misfolded or truncated proteins [38]. The primary function of the mitochondrial proteases is to engage and process unprocessed, misfolded, and damaged polypeptides to maintain mitochondrial homeostasis. Mitochondrial proteostasis is brought out by protein degradation, partial processing with chaperonic rearrangements, or even relocalization of proteins to their accurate location within the mitochondria [34]. Mitochondrial proteases have pleiotropic functions that include protein import, quality control, protein processing, phospholipid trafficking, ribosome assembly, OXPHOS assembly, MCU complex assembly, mtDNA stability, mitophagy, apoptosis, and hypoxic response [4]. It is quite intriguing to understand the roles that mitochondrial proteases may play to influence varied cellular responses and might aid or abet a plethora of diseases that may range from cancer and neurological disorders to aging [39,40,41,42,43]. Proteostasis imbalance can be brought by a reduction in the capacity of protein folding and excessive protein aggregates. Mitochondrial unfolded protein response (mtUPR) generates a protective response against this imbalance [44]. Hetero-dimer of CHOP and C/EBPβ, family of CCAAT/enhancer binding proteins, activates the transcription of mtUPR responsive genes that includes genes encoding mitochondrial proteases YME1L1 and MPPβ [45]. These genes contain a CHOP element in their promoters. Due to the lack of CHOP element in both Afgl32 and Spg7, these genes are not involved in mtUPR [45] although they play key role in mitochondrial protein folding.
Mitochondrial Co-translational Protein Quality Control
AAA protease complexes are responsible for maintaining the mitochondrial quality control that includes homo- or hetero-oligomeric m-AAA proteases [30]. All 13 mitochondrial encoded OXPHOS proteins are co-translationally processed by AFG3L2 after the insertion of these nascent chains into the IMM by OXA1L/OXA1 (coding insertase enzyme) [38]. Genetic interaction of AFG3L2, OXA1, and F1F0 ATP synthase (complex V of OXPHOS), well documented in budding yeast Afg3l2p, regulates co-translational proteostasis that is quintessential in the maintenance of organelle homeostasis and affects the bioenergetics of a cell. Kah Ying Ng’s work has used MT-ATP6 as a representative substrate to understand this co-translational quality control and delineate the steps of regulation in MT-ATP6 pathogenic variants [38]. AFG3L2 is involved in the quality control of OXA1L-mediated MT-ATP6 insertion in IMM. Loss of function of AFG3L2 affects the mitochondrial morphology and simultaneously critical pathways leading to mitochondrial gene expression impairment.
Protein Aggregates
Many neurodegenerative diseases result from protein misfolding leading to aggregation. m-AAAs help in keeping a balance by maintaining the proteostasis. For example, in a genome-wide RNAi screen, AFG3L2 came up as a gene involved in the suppression of mutanthuntingtin protein accumulation in the mitochondria, thus indicating therapeutic potential [17].
Steroid Synthesis
StAR overload response (SOR) is the process of enrichment of mitochondrial proteases and their transcripts by steroidogenic acute regulatory protein (StAR) expression. StAR regulates steroid synthesis in adrenal cortex and gonads by activating the CYP11a1/P450scc enzyme in the OMM. StAR expression also increases the gene transcription of LON and AFG3L2/SPG7 [46, 47]. Excessive StAR production leads to a sequential degradation process of StAR, due to overload, in the OMM by LON followed by in the IMM where it is degraded by LON and AFG3L2/paraplegin. This avoids SOR in the mitochondrial matrix [47].
Functions Related to Expression
AFG3L2 is ubiquitously expressed. It is evident from previous studies that Afg3l2 is expressed throughout the mouse brain corroborating its essential role in the function of the neurons [19]. Further enhanced expression is observed in the large cell body containing neurons that include brainstem motor neurons, mitral cells of olfactory bulb, pyramidal cells of hippocampus and neocortex, Purkinje, and deep nuclei cells of cerebellum [19, 48]. However, Afg3l2 expression patterns are not strictly related to the occurrence of SCA28 or SPAX5 development [19]. So, it can be predicted that there might be unidentified proteins regulated by AFG3L2 which can be a participant to the SCA28 or SPAX5 pathophysiology. Both dominant heterozygous and recessive homozygous mutations of AFG3L2 will lead to depletion in AFG3L2 protein. Loss of AFG3L2 causes mitochondrial network fragmentation [49] affecting mitochondrial anterograde transport [8] but is incapable of causing any human disease [49]. AFG3L2 is highly expressed in Purkinje cells [50] where its deficiency causes SCA28 or SPAX5 [51]. It is also expressed in the neighboring Bergmann glial cells where it plays an important role in glutamate homeostasis in the synaptic and peri-synaptic extracellular environment [52]. Ceftriaxone is a β-lactam antibiotic promoting synaptic glutamate clearance. It upregulates glutamate receptor EAAT2 in the astrocytic glial cells and ameliorates ataxia in heterozygous mutated AFG3L2 by inhibiting glutamate excitotoxicity and creating healthy Purkinje cell and glial connections [53]. The neuron-glial cross-talk is well reported where cytodifferentiation of Bergmann glial cells proceeds in correlation to the cytodifferentiation of Purkinje cells [54]. The work of Rugarli laboratories has clearly depicted the importance of Bergmann glial cells in ataxia. Bergmann glial cells are radial astrocytes neighboring to the Purkinje cells in the cerebellum that help in clearing the glutamate toxicity at the synaptic clefts of Purkinje cells and Bergmann glial cells. This is primarily performed by EAAT1, a glutamate-aspartate transporter, and EAAT2, a sodium ion-dependent glutamate transporter. AFG3L2 deficiency in these regions has shown mitochondrial morphological changes like fragmentation but no OXPHOS dysfunction [52]. Lack of AFG3L2 also upregulates a necroptotic factor called ZBP1 leading to neuroinflammation [52, 55] and metabolic stress responses. It further affects electrophysiological balance in Purkinje cells due to increased Ca2+ influx causing dark cell degeneration and neuronal death of Purkinje cells [53] as a secondary effect. Thus, Purkinje cells show reduced dendrite formations and the absence of firing or excitation during depolarization. Bergmann glial cell is an active participant involved in AFG3L2 deficiency-related neurological disorders (Fig. 3).
Biological pathways that are affected due to mutations and dysfunction of AFG3L2 causing SCA28. (a) The mutation of AFG3L2 is responsible for increasing the neurotropic factor ZBP1 that in turn causes neuroinflammation. (b) MCU and EMRE, two complexes found in the mitochondrial inner membrane, can be disrupted by dysfunctional AFG3L2, which leads to disruption of electrophysiological balance by releasing excess Ca2+ from the mitochondrial matrix to the cytosol. (c) Mutations in AFG3L2 generate an accumulation of misfolded proteins in the mitochondrial matrix followed by the activation of OMA1 protease which enhances the OPA1 processing to its short form and causes the mitochondrial fragmentation. On the other hand, the Complex IV of electron transport chain (ETC) is hampered because of AFG3L2 mutation. (d) The dysfunctional AFG3L2 inhibits the assembly of Complex I and Complex III of ETC that finally leads to the disruption of axonal transport. (e) The defective AFG3L2 is responsible for the disbalance of axon glial cross talk due to insufficient neurofilament. PC, Purkinje cells; BG, Bergmann glial
Maltecca and co-workers have shown that Afg3l2 missense and null mutant mice have no effect on mitochondrial protein synthesis but impact on mitochondrial energy metabolism by impaired respiratory complex I and III activity due to inadequate assembling of these complexes. This is a result of swollen and giant mitochondria with damaged cristae generated in the vacuoles of Purkinje cells as well as cell bodies of the spinal cord and dorsal root ganglia that are located near to nucleus and cell membrane [56] affecting axonal transport (Fig. 3). They have further used carbonyl formation as a marker to emphasize the importance of oxidative stress in Afg3l2 mutants [57]. NADH dehydrogenase 1 (ND1) that causes the initiation of complex I formation is degraded in AFG3L2-dependent manner [58]. Early neuronal development in the demyelinated axons involves Nrg1-III signaling via ErbB receptors [59] while post-myelination axonic development involves phosphorylation of neurofilaments (NF) utilizing the kinase-phosphatase cycles [60]. Afg3l2 mutants impact the axon-glial cross talk because damaged axons affect myelination, result in insufficient NF phosphorylation and also cause glutamate excitotoxicity in glial cells [56, 61].
Mitochondrial proteotoxicity is a causative agent of SCA28. Heterozygous M665R mutation of Afg3l2 in a mice knock-in model showed enhanced Purkinje cells firing and changes in the mitochondrial energy metabolism that includes a reduction in membrane potential, oxygen consumption, and ATP synthesis leading to mitochondrial fragmentation. The fragmentation was due to the presence of excess OPA1 short forms [62]. Homozygous M665R mutation is lethal. Chloramphenicol, a mitochondrial protein synthesis inhibitor, was reported to be able to reverse these mitochondrial morphologies like fragmentation in mouse embryonic fibroblasts (MEFs) generated from homozygous M665R mutation of Afg3l2 in mice. These morphologies include mitochondrial network formation and shapes. To date, one epigenetic case study on monozygotic twins has revealed that hypomethylation of AFG3L2 in one of the twins maintains health while changes in methylation at multiple loci of AFG3L2 called as differential methylation on the other caused myocardial infarction [63]. However, this observation needs further exploration.
AFG3L2 and Cellular Respiration
In general, AFG3L2 acts as a sensor to maintain organelle fitness by regulating mitochondrial proteostasis. This is done by coordinating the OxPhos complex assemblies [12]. Under stress conditions, membrane potential is compromised leading to OMA1 activation that cleaves OPA1 required for mitochondrial fusion. However, mitophagy due to inhibition of anterograde transport in AFG3L2-depleted cells confirms that it is a result of mitochondrial fragmentation/fission [8]. Rugarli laboratory has successfully shown the role of Afg3l2 in Purkinje cell survival by supporting mitochondrial protein synthesis. Deletion of AFG3L2 clearly reduced only the levels of mt-encoded CYTB, COX1, COX3, and ND2 in the brains but not the levels of nuclear-encoded COX4 and NDUFA9 that are mostly related to the electron transport chain [50]. Respirasomes are supercomplexes of OxPhos present in mitochondrial inner membrane. Truncated COX1 can still interact with AFG3L2 and form unstable supercomplexes. AFG3L2 abundance or depletion can regulate the stability of the truncated COX1 and its ability to generate stable or unstable respirasomes respectively [64].
Inefficient cell respiration due to loss of AFG3L2 cause reduced cell proliferation and defective biogenesis of cell respiratory proteins, elevated expression of OMA1, and loss of paraplegin [65]. It mainly affects the assembly and hence the function of Complex IV of electron transport chain which is the rate-limiting step of oxidative phosphorylation. This may lead to an uncoupling effect thus reducing the oxygen consumption, affecting the ATP synthesis and activating apoptosis [66] (Fig. 3). Deficiency of cytochrome c oxidase (Complex IV) causes aging and degenerative disorders. At the molecular level, reduced cytochrome c oxidase affects mitochondrial membrane potential, ATP synthesis, calcium uptake, and ROS production. Caveolin-1 acts as an important interacting protein of AFG3L2 and promotes translocation of both these proteins to the mitochondria especially after enhanced oxidative stress in fibroblasts [67]. Curbing their interaction with mutant AFG3L2 protein affects Complex IV formation after oxidative stress. Loss of caveolin-1 inhibits mitochondrial translocation of AFG3L2 thus affecting mitochondrial protein quality control, collapse of OXPHOS, and lowering of ATP production [67].
A study on primary MEFs and primary cortical neurons by Bettegazzi and coworkers in Afg3l2-knock out (KO) mice showed that in spite of mitochondrial dysfunction leading to increased ROS production, the cells overexpressed antioxidant peroxiredoxin 3 and had higher glutathione levels after initial vulnerability of few days. This evidently maintained the cell viability as well as the quality control of the cell and can be utilized in designing therapeutic targets against aging cells found in several neurological disorders [68].
Mitochondrial Stress Response
m-AAA mutations in AFG3L2 or SPG7 lead to proteotoxic trigger due to defective mitochondrial mRNA synthesis followed by inhibited nascent polypeptide chain quality control. This leads to a stress response affecting the inner membrane morphology and homeostasis of ribosome [69]. Mitochondrial stress response is handled by i-AAAs and m-AAAs differently. AFG3L2 is the major protease involved in the i-AAA and m-AAA coordinated processing of OMA1 consequently controlling the distribution of the long (L-) and short (S-) isoforms of OPA1 [70]. i-AAAs YME1 and OMA1 separately or cooperatively control the OPA1 processing thus maintaining the long (L-) and short (S-) forms of OPA1 and this further maintains proteostasis by balancing mitochondrial fission and fusion [11, 70]. L-OPA1 is essential for mitochondrial fusion. The two forms of OPA1 is balanced based on normal and stress conditions under ATP replete or ATP depleted conditions respectively. This also affects the lamellar cristae morphology, OXPHOS function and protein synthesis (Fig. 3). Richter U and co-workers, observed for the first time, the connection between OPA1 processing and protein synthesis in mitochondria is linked to ribosomes [69]. They showed that AFG3L2 dysfunction affecting protein quality control triggers stress response by OMA1 mediated S-OPA1 formation and leads to ribosomal decay [69]. Quantitative overexpression of wild AFG3L2 or AFG3L2 E575Q mutation (proteolytic mutant inhibiting homo-oligomerization but maintaining AAA domain function) reduces OPA1 processing and mitochondrial ribosomal protein synthesis in MT-ATP6 m.9205delTA mutation [69, 71]. AAA domain of AFG3L2 with chaperonic function has epistatic suppression effect on MT-ATP6 m.9205delTA non-stop mutation during heat shock stress [69]. AFG3L2 mutations lead to enhanced OMA1-dependent S-OPA1 formation and mitochondrial fragmentation [72,73,74] that ultimately causes neurodegeneration. Overexpression of AFG3L2 in these mutants does not rectify the aberrant protein synthesis [69].
Substrate Identification and Processing
Specialized mechanisms are involved in substrate-specific identification, recruitment and degradation/processing of substrates but these mechanisms still largely remain unclear [31]. Interestingly, given their role in diverse cellular activities, these AAA + proteases recognize both highly specific sequences like the degron sequences and residue patterns as well as accessible sequences in unstructured regions thus performing both targeted protein degradation and untargeted degradation. Degron sequences and residue patterns following scissile peptide bond (a covalent chemical bond) region are the two ways by which m-AAA proteases maintain their specificity. For example, hydrophobic and small polar residues in P1 position at N-terminal side of substrate MrpL32, a mitochondrial ribosome act as a degron that interacts with AFG3L2 for degradation [29, 75]. The loss of AFG3L2 (m-AAA) and YME1L (i-AAA) results in mitochondrial fragmentation and impairs cell respiratory biogenesis [65]. Accurate mechanisms by which AAAs precisely identify the degron sequences still largely remain unexplored for majority of their substrates.
MCU Complex Assembly and Calcium Homeostasis
m-AAA like AFG3L2 are also involved in mitochondrial calcium uniporter (MCU) complex assembly [4]. Mutations of AFG3L2 affect this assembly leading to Ca2+ overload, increased reactive oxygen species (ROS) production due to elevated cellular respiration thus releasing proapototic factors like cytochrome C into the cytosol. Purkinje cells are prevalently affected in ataxias due to the imbalance in their calcium ion reservoir which in general is linked to calcium channels, calcium interacting protein, calcium-related phosphatases, and kinases [76]. Mitochondria, due to its close proximity to both endoplasmic reticulum (ER) and cell membrane promotes calcium influx from ER and cell membrane into the mitochondria, thus act as a calcium buffering system. At neuronal synapses, this affects the neurotransmitter release especially in Purkinje cells that cause an elevated firing rate [4].
Ca2+ homeostasis is majorly affected in Purkinje cells that have highly branched dendrites and receive excitatory inputs through glutamate stimulated receptors AMPA and mGluR1 [77]. This excitation increases the Ca2+ uptake in these cells. Lack of AFG3L2 not only causes mitochondrial fragmentation, it possibly keeps mitochondria available and functional in the soma of the Purkinje cells close to ER, thus making less mitochondria and ATP available in the axons and dendrites [61]. This restricts the ATP-dependent Ca2+ uptake in these major excitatory regions. This may lead to glutamate toxicity leading to apoptosis of these cells causing neurodegeneration.
LACE1, the human homolog of yeast Afg1 (mice Afg3l1), deletion causes apoptotic resistance while LACE1 overexpression enhances apoptosis [78]. It helps in the mitochondrial translocation of p53. However, such evidence is not yet recorded in AFG3L2. AFG3L2 deletion causes mitochondrial fragmentation while deletion of both AFG3L1 (another m-AAA complex component) and AFG3L2 causes oligodendrocyte/glial death due to demyelination and motor dysfunction including hair graying [49]. Konig and Rugarli’s work from Langer laboratory found that loss of m-AAAs generates constitutively active MCU-EMRE channels that cause calcium overload in mitochondria affecting permeability and leading to cell death [41, 54].
MCU complex includes pore-forming MCU unit, regulatory proteins MICU1 or MICU2, and MCU interacting protein EMRE [41, 79,80,81]. The active MCU includes all of these components, and its assembly is brought about by m-AAAs. In the absence of the regulatory protein (MICU1/MICU2) or its interaction with EMRE, that is required prior to the complete complex assembly, MCU and EMRE assemble into a constitutively active complex that disrupts calcium homeostasis and brings in apoptosis [41]. This can be the reason for neurodegeneration and muscle disorders. MCUb in mice is a variant found as a dominant negative form [82]. It is reported that the absence of AFG3L2 increases the cytosolic calcium influx in the Purkinje cells causing ataxia due to constitutively active MCU-EMRE assembly [4]. It is noteworthy to mention that MICU1 deletion results in the development of ataxia and muscle weaknesses [80]. Excitatory increase in mitochondrial Ca2+ causes SCA28 by Purkinje cells apoptosis [80]. EMRE acts as a novel substrate of AFG3L2, and its regulated proteolysis maintains Ca2+ homeostasis. AFG3L2/paraplegin duo degrades unassembled EMRE utilizing ATP breakdown [6]. The study of regulation of Ca2+ homeostasis by AFG3L2 can identify novel MCU inhibitors as prospective therapeutic intervention against the apoptotic process. While in one hand m-AAA regulate MCU complex assembly and hence the mitochondrial calcium uptake, on the other hand, they regulate mitochondrial protein synthesis and further influence ROS production and cellular death by apoptosis [4]. Maintenance of proteostasis is a multi-step process including many mechanisms, and hence, inhibition of mitochondrial Ca2+ uptake may not lead to Purkinje cell survival as per the studies undertaken by Langer laboratory [4, 41].
Maltecca and co-workers have pinpointed that loss of Afg3l2 in MEFs causes primarily respiratory dysfunction leading to mitochondrial fragmentation and enhanced OPA1 processing that affects calcium homeostasis. The lack of calcium diffusion into mitochondria is clearly due to the impaired cross-talk between the ER and the mitochondria that can be retrieved by OPA1 or MFN1 overexpression. However, this approach also exhibit continuing respiratory defects [61]. Later, Maltecca’s laboratory and Tempia’s laboratory have proven in SCA28 cells that OMA1 hyperactivation followed by increased processing of OPA1 causes defective mitochondrial fusion due to the accumulation of mtDNA-encoded proteins [62, 83]. This also reduced the mitochondrial calcium uptake.
Ataxia
National Ataxia Foundation of America reports that approximately 150,000 Americans are affected with sporadic or hereditary ataxia. People of any age or sex can suffer from ataxia, a slowly progressive neurological disorder. Ataxia is an uncontrolled movement disorder caused by the improper functioning of the nervous system. There are three kinds of ataxia: vestibular ataxia, spinocerebellar ataxia, and proprioceptive or sensory ataxia [84]. These are also categorized as vestibulocerebellar, cerebellar motor, and cerebellar cognitive syndromes based on their neuroimages and anatomical findings [85]. Vestibular ataxia develops due to impaired functioning of vestibular system, which senses movement of head and help maintain balance and spatial orientation. It involves the inner ear and the ear canals. Abnormality in nerves of vestibular system manifests the following symptoms: blurred vision, nausea and vomiting, problems with standing and sitting, trouble in walking, virtigo, or dizziness [86]. Spinocerebellar ataxia develops due to impaired functioning of cerebellum or spinal cord, which helps maintaining balance and coordination of body movements [87]. Symptoms of cerebellar ataxia include headache, changes in voice, slurred speech, fatigue, muscle tremors, dizziness, trouble walking, and wide gait. Proprioceptive or sensory ataxia develops due to impaired functioning of the nervous system that is outside of the brain and spinal cord, which helps maintain touch sensation of the skin. Symptoms of sensory ataxia include difficulty touching finger to nose with closed eyes, inability to sense vibrations, trouble walking in dim light, and walking with a heavy step [84].
Inherited ataxias can be autosomal dominant or recessive, X-linked, and episodic ataxia [85]. While dominant ataxia is easily diagnosed with prominent phenotypes, recessive ataxia shows a high heterogeneity in its phenotypic expression and X-linked ataxia are best identified from trios studies. Recessive ataxia is often initiated in childhood or early adulthood [85]. Strupp laboratory categorizes the phenotypic expressions of autosomal recessive cerebellar ataxias (ARCAs) into six categories but AFG3L2 related SPAX5 falls under metabolic or mitochondrial syndrome [85]. Difficulties in diagnosis and management are more evident in identification of autosomal recessive ataxia where combined results of next generation sequencing and trios studies help in accurate diagnosis and detection [88]. In the past 4 years, 15 cases of early onset cerebellar ataxia have been diagnosed in Kasturba Hospital, Manipal, India. Indian prevalence in cerebellar ataxias is reported as 4.8 to 13.8 in 100,000 individuals [89]. SCA28 is a rare type of ataxia, and AFG3L2 has been shown to be involved with this disorder in several studies across the world [22, 29, 83, 90,91,92]. However, the molecular mechanisms are yet to be deciphered.
So far, some mechanisms related to AFG3L2 mutagenesis that contributes to SCA28 pathophysiology have been reported. Being a m-AAA protease in the inner membrane of mitochondria, AFG3L2 is involved in mitochondrial proteostasis and cellular respiratory biogenesis [65]. Loss of these AAA proteases can affect protein synthesis and cellular respiration [4]. It is highly expressed in GABA-ergic Purkinje cells of cerebellar cortex [51] and involved in the axonal anterograde transport of mitochondria [8]. The neuronal interactome of AFG3L2 includes paraplegin, PINK1, EMRE, and MAIP1 as the prevalent interaction proteins [4]. Since AFG3L2 demonstrates both autosomal dominant and recessive mutational inheritances, it adds on to the diagnostic dilemmas at both allelic and phenotypic aspects of SCA28 or SPAX5 development and gives added challenges to genetic counselling.
AFG3L2 and Its Related Mutations in Ataxia
AFG3L2 is a mitochondrial inner membrane zinc metalloprotease, essential for axonal transport of mitochondria and neuronal development [4], required for paraplegin and PINK1 maturation [93] as well as has chaperonic function of ATPase domain [11]. SPG7 mutations cause autosomal recessive hereditary spastic paraplegia (HSP type 7) affecting Complex I activity and oxidative stress [94, 95]. While autosomal dominant AFG3L2 mutations cause SCA28, homozygous recessive mutations lead to SPAX5 [12, 19, 24]. Disease-relevant AFG3L2 mutations are localized in four hotspots of which three hotspots categorized as autosomal dominant mutations are found in three inter-subunit interfaces while all recessive mutations are found in the peripheral active sites of protease ring [36] (Fig. 1). These mutations derange the nucleotide dependent substrate translocation affecting the substrate interacting non-conserved regions. All protease domain recessive mutations except N435T condense at the central protrusion [36]. However, these spinocerebellar ataxia of autosomal recessive types abbreviated as SCAR are difficult to identify and their mechanisms remain less explored and elusive [96]. The domain structure of AFG3L2 listed in Table 1 and Fig. 1 gives us a clear depiction of how domains can be related to the neurological disorders. We can see that mutations in the catalytic domain cause optic atrophy or OPA12 while the pro-peptide, inter-TM1 (Trans-membrane region 1), IMS (Inner membrane space), and proteolytic domain mutations are mainly responsible for SCA28. SPAX5, being a recessive phenotype, is rarely expressed and hence is less documented, but mutations are found to be scattered in various domains inter-TM1, IMS, and proteolytic domains.
Mutations in Spinocerebellar Ataxias
Spinocerebellar ataxias (SCA) or autosomal dominant cerebellar ataxia (ADCA) are rare autosomal dominant progressive neurological disorders characterized by gait imbalance and motor incoordination of hand, speech, and eye movements—a primary cerebellar dysfunction. By 2010, although over 30 SCA genes were identified that are related to these disorders, the cellular and molecular events have not been completely deciphered yet [97]. 18p whole arm translocation was reported to be causing dystonia with symptoms of rigid postures and muscle contractions [98]. In 1999, AFG3L2 was mapped at location 18p11, and further, the locus of SCA was identified in 2006 where specifically 18p11.22-q11.2 was designated as SCA28 [24,25,26]. Haploinsufficiency has also been reported to cause SCA28 [99]. A detailed list of mutations in AFG3L2 and their relation to domain structures can be seen in Table 1 and Fig. 1. The first case of heterozygous deletion of AFG3L2, a causative reason for SCA28 due to haploinsufficiency which has multiple genomic anomalies, was reported in 2014 [100]. However, Lohmann laboratory studying SPAX5 related mutations had previously stated that heterozygous AFG3L2 mutation causes SCA28 [101]. AFG3L2 mutations, although are related to cerebellar ataxias, does not exhibit polyglutamine repeats like the majority of SCA genes [99]. Patients with no signs of prevalent SCA types are screened for SCA28 and symptoms include oculomotor signs of a very slowly progressive ataxia [25]. Cagnoli and co-workers [102] reported six missense mutations of AFG3L2 in nine unrelated index cases from 366 European families having ADCA (autosomal dominant cerebella ataxia). Similarly, in a Taiwanese cohort, only one patient was reported for AFG3L2 mutation among 133 cerebellar ataxia patients. In another ADCA cohort study, Jia et al. [91] did not find any AFG3L2 mutations from 67 patients. In 2013, a study on the lymphoblastoid cell lines of four SCA28 patients revealed 66 genes with statistically different expression patterns [103]. This was the first genome-wide analysis that identified 35 upregulated and 31 down-regulated genes that were categorized into five functional categories that are related to cell proliferation, programmed cell death, oxidative stress response, cell adhesion, and chemical homeostasis. The differential expression is related to phenotypes of SCA28 that include impaired growth, increased G0/G1 phase cells, increased apoptosis, enhanced lipid peroxidation, and increase in mitochondrial regulators TFAM and DRP1 but no alteration in ROS levels and respiratory chain activity.
AFG3L2 gene with 17 exons encodes a 797 amino acid protein. By 2010, AFG3L2 gene mutations was identified to cause SCA type 28 [90]. It has been reported that exons 15 and 16 are the mutational hotspots for AFG3L2 for causing SCA28 [23, 102]. AFG3L2 also has selective overexpression in the Purkinje cells and many AFG3L2 dominant mutations as discussed by Di Bella D and coworkers’ results in SCA28 [51, 87]. Y689H substitution in the M41 peptidase domain was reported as pathogenic and to cause SCA28 [87]. First missense mutation in exon 4 (not the mutation hotspot) of an African origin patient, V191I [c.571G > A], was identified in a 68-year-old patient [104]. But, it could not be identified whether this was a founder mutation. A novel heterozygous partial AFG3L2 deletion of exons 14 to 16 was reported to cause loss of cerebellar function with ptosis [105]. The disease mechanism was related to ubiquitin and p62 nuclear inclusions and haploinsufficiency. Thirteen missense mutations of AFG3L2 mostly in exon 16 have been identified till date, but cases are rare in Western countries [92]. However, all mutations discovered and documented so far are mostly in Caucasian populations. A novel G671R [c.2011G > C] mutation that was located in a highly conserved region of AFG3L2 gene in five patients of a Hungarian family showed similar characteristics to previously identified cases with no cognitive impairment [106] and included pathogenic mutations G671R [c.2011G > A] and G671W [c.2011G > T] [102, 107]. A rare compound heterozygous mutations of AFG3L2, Y616C [c.1847A > G], and V723M [c.2167G > A] caused ptosis in his son as well as his asymptomatic single heterozygous mutated mother [103]. This mutation can act as a pre-diagnostic marker for future case detections. Such compound heterozygous mutation adds more to mitochondrial dysfunction both structurally and phenotypically. Heterozygous loss-of-function mutations in AFG3L2 cause SCA28 while homozygous missense mutation Y616C [c.1847A > G] in AFG3L2 causes early onset spastic ataxia-neuropathy syndrome also known as SPAX5 [23]. p.A484P variant of AFG3L2 segregated with STIP1 homology and U-Box containing protein 1 (STUB1) variant (Y49C) that causes SCA16 and SCA48 [108]. A Taiwanese patient showing multiple heterozygous mutations, i.e., [2167G > A]; [V723M] (c.[1894C > T]; [R632*], suffered from sporadic and slow progressive SCA [96] and represented a new subtype. Maltecca and co-workers [99] have identified two mutations—homozygous missense and homozygous null—that caused lethality in mice models due to impaired axon development in both CNS and PNS delayed myelination, and weak axonal radial growth [56]. In Spg7 deficient mice, Emv66 mutants of AFG3L2 show exacerbating axonopathy and severe neuromuscular defects in hetero- and homozygous conditions respectively with loss of PCs and parallel fibers [109]. Heterozygous P688T [c.2062C > A] mutation at highly conserved site in exon 16 in three patients of a family for the first time reported non-neuronal skeletal muscle fiber atrophy type I along with SCA28 phenotypes [110].
Mutations in Other Ataxias
Interestingly, SCAR mutations cause spastic ataxia-neuropathy syndrome or SPAX5 [23]. Purkinje cell-specific deletion of Afg3l2 leads to mitochondrial fragmentation due to impaired mitochondrial ribosome assembly and protein synthesis [50]. However, ubiquitous AFG3L2 deletions lead to delayed myelination causing lack of axonal development and a severe neurological phenotype [56].
Tyrosine phosphorylation of paraplegin by AFG3L2 helps in paraplegin processing and inhibition of this processing can cause ROS production [111]. On the contrary, the Q688 variant of paraplegin can bypass the tyrosine phosphorylation regulation of AFG3L2 [112]. Another type of SCAR found by Calandra and co-workers includes two novel AFG3L2 heterozygous mutations (W128* and R695G) that cause spastic ataxia with unique eye-of-the-tiger pattern that can be related to iron deposition and pantothenate kinase-related neurodegeneration that affects CoA synthesis pathway [18]. Long-term observation will be required to see the development of SCA28 phenotype if any. Yeast homolog Yta10 of AFG3L2 is reported to mislocalize during hypoxia [113] and triggers the question about the role of AFG3L2 in the proteostasis of mitochondrial proteins and the onset of SCA28.
Heterozygous Y689H and G671W mutations of AFG3L2 cause progressive external ophthalmoplegia due to somatic mtDNA disbalance affecting fibroblasts and muscles of the eye [107]. AFG3L2 was identified as one of the brain-expressed longevity genes [114], and further, SNPs in AFG3L2 were identified relating to aging [115]. Heterozygous R468C mutation along with heterozygous SPG7 deletion leads to aberrant OPA1 processing. Since OPA1 is involved in mitochondrial fusion, this mutation further causes mitochondrial fragmentation. Phenotypic characterization is expressed as early-onset optic atrophy associated with Parkinsonism (L-Dopa responsive) and spastic ataxia [22]. G337E [c.1010G > A] mutation of AFG3L2 causes aberrant processing of OPA1 and OMA1 leading to optic atrophy [116]. Homodimer of AFG3L2 causes better OPA1 processing and continues this process even in the absence of heterodimer with paraplegin or presenilins-associated rhomboid-like protein (PARL) [117]. Involvement of OPA1 processing with various dimer formations of m-AAAs provides flexibility in mitochondrial biogenesis in a tissue/ function-specific manner and therefore improves the quality of life [30]. Thus, AFG3L2 clearly plays a vital role in the prevention of neurodegeneration in the cerebellum by regulating mitochondrial proteostasis and restricting apoptosis. More investigation is required to understand this mechanism.
Diagnosis, Management, and Treatment of SCA28
Diagnosis and management of cerebellar ataxia needs a multidisciplinary approach with neuroimaging, anatomical studies, genetic tests, motor tests, and molecular/biochemical studies playing crucial roles [85]. Since SCA28 is a slowly progressive, young-adult onset ataxia that in most of the cases may take decades to be identified, the diagnosis usually is restricted to a proband whose either parent was affected and hence can be diagnosed by genetic testing via exome sequencing for the typical pathogenic variant as discussed in this review article. These tests necessarily need to include multigene panel and comprehensive genetic analysis. Till date, exome or genome sequencing is not yet available routinely for SCA28 patients [118]. Clinical exome sequencing is the most efficient and economical way to diagnose the rare pathogenic variants and to discover likely variants of AFG3L2 [119]. The presence of the AFG3L2 mutations through family history also becomes challenging due to delayed diagnosis or the fact that probably it remained undetected due to the early demise of the parent [118, 120]. Neuroimaging may sometimes show a shrunken cerebellum [118] but mostly show a normal neuroimaging in MRI/CT scan [121]. Hence, a keen investigation with family history, physical examinations, genetic testing, and neuroimaging might be useful in diagnosing this disease. Very recently, c.2167G > A; V723M variant is designated as a likely pathogenic variant of AFG3L2 that is related to myopathy, respiratory chain complex defects, and ataxia [122] in a single patient who is also affected by congenital pituitary hormone deficiency and deafness due to involvement of other mutated genes as well.
There is a complete lack of treatment for SCA28 primarily because of its slow progression and difficulty in timely diagnosis. Hence, it is of utmost importance to manage this disorder well with timely interventions whenever diagnosed. These interventions include ambulatory aids, stretching exercises, and physical therapy; ergonomic home adaptations; speech and psychological therapies; weight control assessment; and gastronomic feeding—all or as many of these as required along with annual assessment [118]. Very recent studies have identified an important variant of the nuclear-encoded AFG3L2 with a SNP affecting mRNA half-life and associated with exercise response phenotypes [123].
Other Clinical Manifestations of AFG3L2
Spastic Ataxia Syndrome (SPAX5)
SPAX5 or spastic ataxia type 5 is a SCAR caused by the homozygous recessive missense mutations of AFG3L2. This syndrome is an early-onset type with cerebellar dysfunction but is characterized by spasticity and epilepsy. Since AFG3L2 mutations affect both homo- and hetero-hexamerization of AFG3L2, it includes the severity of both SCA28 and spastic paraplegic ataxia due to the involvement of SPG7 in hetero-hexamerization. AFG3L2 dysfunction can severely damage mitochondrial functioning causing massive fragmentation and reduced calcium uptake [83]. The mutation specifically at Y616C [c.1847A > G] of AFG3L2 leads to phenotypically different manifestations from SCA28 and causes spastic ataxia [23]. However, homozygous mutations such as W128, I216F, and I705T also cause SPAX5 and are listed in Table 1, and compound heterozygous mutation can cause SPAX5 too. Compound heterozygous Y616C [c.1847A > G] with p.V723M [c.2167G > A] mutation [23] also is reported to cause SCAR. SPAX5 manifestation shows a severe reduction in AFG3L2 expression in a case study with biallelic compound heterozygous p.V212Gfs*4 [c.634dupG] with p.V723M [c.2167G > A] mutation [124]. The severity of this syndrome may surely affect other genes since it is the outcome of dysfunction of m-AAA proteases. Homozygous M625I [c.1875G > A] mutation is reported as a rare mutation of AFG3L2 related to progressive myoclonus epilepsies [125, 126].
Parkinson’s Disease
Unique frameshift AFG3L2 mutation, c.1958dupT in exon 15, causes mild Parkinsonism with cognitive decline, cerebellar ataxia, bradykinesia, and polyneuropathy [127]. The direct relation of this mutation with the phenotype of the patient is currently unclear, and this being a case study shows a weak association with PD. However, AFG3L2 haploinsufficiency and surely not the dominant-negative effect of missense AFG3L2 mutation reported in SCA28 is likely the reason for this pathogenesis [127]. AFG3L2 aids in PINK1 maturation by generating a 52kD processed fragment that can localize in IMM and hence may indirectly be linked to PD. PINK1, a protein associated with the elevation of autophagy, is synthesized in cytosol and interacts with alpha-synuclein [128]. Cofilin, an actin-binding protein, mediates PINK1/PARK2-dependent mitophagy [126]. PINK1 accumulation due to dysregulation of AFG3L2 and mitochondrial membrane potential [129] can affect this process leading to cancer progression. PINK1 undergoes a stepwise degradation from cytosol to mitochondrial matrix that is controlled by MPP, PARL, AFG3L2, and ClpXP respectively [93]. Under stress conditions, PINK1 which is partially cleaved by mitochondrial processing peptidase (MPP) is transported to IMM and undergoes presenilin-associated rhomboid-like protein (PARL) and AFG3L2-dependent processing forming a 52 kD fragment [15, 130]. During membrane depolarization or ATP depletion, the absence of PINK1 cleavage by MPP recruits uncleaved PINK1 to the outer mitochondrial membrane (OMM). PINK1 further recruits Parkin, a E2 ubiquitin ligase causing the mitochondrial fragmentation of damaged mitochondria [3]. A well-known fact today is that the loss-of-function of Parkin leads to PD, both due to the absence of PINK1-Parkin interaction and alpha-synuclein accumulation [131, 132]. Whether AFG3L2 mutations causing SCA28, SPAX5, or optic atrophy can cause additional risk of PD by generating altered PINK1 cleavage is an intriguing aspect to test. Mendelian disease mapping in a family by pedigree analysis must be performed to establish causation. AFG3L2 loss leads to tau hyperphosphorylation [8] although the mechanism behind its relation to AD is not yet studied. Mutation in PARK8 gene locus encoding LRRK2 plays a vital role in PD [133].
Mitochondrial Disease
In a case study including a consanguineous family, c.1714G > A A572T mutation on AFG3L2 was identified in the first-ever exome sequencing analysis to be involved in a new mitochondrial phenotype that caused high lactate and respiratory chain defects [134]. Cerebellar atrophy was noticed in the neuroimaging. In a detailed study, this mutation was seen to cause microcephaly with early onset seizures and involves basal ganglia that are not noticed in other mutation types. This has further led to refractory epilepsy and death. This study emphasizes that this AFG3L2 mutation can be added as a genetic marker along with neuroradiological and biochemical spectrum for a rare mitochondrial disease with neurodegenerative phenotype.
Optic Neuropathy and Other Eye Associated Ataxias
Optic neuropathy or dominant optic atrophy (DOA) is a dominant and inherited mitochondrial disease majorly caused due to mutations in OPA1. The cleavage of this protein is dependent on i-AAA YME1L and m-AAAs AFG3L2 and paraplegin. SPG7 mutations are more often related to this disease. Other mutations include OPA3 [135, 136], OPA5 [137], and WFS1 [138]. c.1402C > T R468C in AFG3L2 was identified as a novel mutation in two patients in a family from two generations who additionally had dyschromatopsia but no sign of either SCA28 or SPAX5 [9, 13, 14]. It is possible that these patients may develop cerebellar ataxia later in their life. In that case, this mutation can act as a pre-diagnostic marker for SCA28 or SPAX5 and surely as a marker for optic neuropathy-related blindness. It will also be interesting to find whether this mutation is present in any SCA28 or SPAX5-affected patients. Eight variants of AFG3L2 responsible for DOA are located in different domains and establish AFG3L2 as a candidate gene for DOA detection [139].
Caporali and co-workers performed an exhaustive study on the role of AAAs in optic neuropathy where their studies showed using both yeast and patient fibroblast that AFG3L2 mutations indirectly affect OPA1 processing, resulting in mitochondrial fragmentation not seen in SCA28 patients. This has been proposed as the crucial mechanism behind the pathogenicity for the studied variants. It clearly summarized 38 AFG3L2 variants in patient cohorts from their own as well as other studies where pathogenic variants related to optic neuropathy were clustered in the ATPase domain affecting either of the functions related with ATP hydrolysis, ATP binding, and substrate interaction. The rest of the variants associated with SCA28 and SPAX5 were clustered in the proteolytic domain [140]. The patient cohort that was studied included 12 different families with familial or sporadic mutations and followed for two to five generations to understand the genetic mechanism. Mutations of AFG3L2 related with DOA are varied and include dominant, recessive, and dominant de novo mutations involved in both sporadic and familial cases and can be pure or syndromic forms. Optic atrophy-12 (OPA12) is an autosomal dominant neurologic disorder caused by AFG3L2 mutation in 18p11.21 location [140]. A heterozygous AFG3L2 mutation together with a homozygous SETX mutation causes AOA2 (ataxia with oculomotor apraxia type 2) with myoclonus [141]. This further corroborates the potential of AFG3L2 as a prognostic and/or diagnostic marker for various mitochondrial diseases and neurodegenerative disorders related to aging.
Conclusion
AFG3L2, a AAA protease, is an ATP-dependent proteolytic complex which is involved in the mitochondrial quality control and protein processing. Two isoenzymes are found in human AAA protease—(i) a complex made up of paraplegin and AFG3L2 which is a hetero-oligomeric complex and (ii) a homo oligomeric complex consisting of AFG3L2. Hereditary spastic paraparesis (HSP) is mainly associated with the dysfunction of paraplegin, whereas mutation of AFG3L2 is involved with primarily causing SCA28. Most of the studies imply that AFG3L2 mutations are majorly linked with autosomal dominant SCA28, and some mutations are involved in developing the SPAX5 or SCAR and optic atrophy. As several mutations are reported in exons 15 and 16 repeatedly, these exonic regions are considered the hotspots of SCA28 pathogenesis. Trios studies for genetic analysis are highly recommended as routine diagnosis during pregnancy and in infant check-up sessions to enable the early detection of the at-risk asymptomatic individuals. Detection of recessive mutations in AFG3L2 would be possible only by genetic testing for cases like a child born to asymptomatic parents, adopted child, paternity issues, and maternity issues in case of surrogate motherhood. As AFG3L2 is mainly associated with the mitochondrial biogenesis and dynamics, its malfunction leads to a variety of neurodegenerative disorders. The effect of the absence of AAA proteases on the metabolic or mitochondrial mechanisms in neuronal dysfunctions is poorly studied. This study explores the future prospects of AFG3L2, as well as its roles in the development of diseases and their therapies. Finding out the molecular basis of the pathophysiology of neuronal disorders should be a major goal to understand the underlying mechanisms. Hence, more studies are required on AFG3L2 and its mutations.
Data Availability
Not applicable.
Abbreviations
- AAA:
-
ATPase associated with diverse cellular activities
- ADCA:
-
Autosomal dominant cerebellar ataxia
- AFG3L2:
-
ATPase family gene 3-like 2
- AMPA:
-
Amino-methyl phosphonic acid
- AOA2:
-
Ataxia with oculo-apraxia type 2
- ARCA:
-
Autosomal recessive cerebellar ataxia
- ANS:
-
Autonomic nervous system
- ATP:
-
Adenosine triphosphate
- CHOP:
-
C/EBP homologous protein
- CNS:
-
Central nervous system
- COX 1/ 2:
-
Cytochrome C oxidase subunit 1/2
- CT Scan:
-
Computed tomography scan
- CYTB:
-
Cytochrome b
- DOA:
-
(Autosomal) Dominant optic atrophy
- EM:
-
Elecron microscope
- EMRE:
-
Essential MCU regulator
- ER:
-
Endoplasmic reticulum
- ErbB:
-
Erythroblastic oncogene B
- GABA:
-
Gamma-aminobutyric acid
- IMM:
-
Inner mitochondrial membrane
- IMS:
-
Inner membrane space
- LACE1:
-
Lactation elevated protein 1
- LON:
-
Protease La
- LRRK2:
-
Leucine-rich repeat kinase 2
- m-AAA:
-
Mitochondrial AAA
- MCU:
-
Mitochondrial calcium uniporter
- MRI:
-
Magnetic resonance imaging
- MAIP1:
-
M-AAA protease interacting protein 1
- MAPK:
-
Mitogen-activated protein kinase
- MEF:
-
Mouse embryonic fibroblast
- mito-DLP1:
-
Mitochondrial dynamin-like protein 1
- MPP:
-
Mitochondrial processing peptidase
- mt:
-
Mitochondrial
- mTOR:
-
Mammalian target of rapamycin
- mtUPR:
-
Mitochondrial unfolded protein response
- ND 1/2:
-
NADH dehydrogenase 1/2
- NDUFA9:
-
NADH ubiquinone oxidoreductase subunit A9
- NF:
-
Neurofilament
- NMR:
-
Nuclear magnetic resonance
- OMA1:
-
Overlapping with m-AAA protease 1
- OPA:
-
Optic atrophy
- OXA1L:
-
Oxidase assembly protein 1 long form
- OXPHOS:
-
Oxidative phosphorylation
- PARL:
-
Presenilins-associated rhomboid-like protein
- PD:
-
Parkinson’s disease
- PINK1:
-
PTEN-induced kinase 1
- PNS:
-
Peripheral nervous system
- ROS:
-
Reactive oxygen species
- SCAR:
-
Spinocerebellar ataxia, autosomal recessive
- SCA28:
-
Spinocerebellar ataxia type 28
- SETX:
-
Senataxin
- SOR:
-
StAR overload response
- SPAX5:
-
Spastic ataxia 5
- SPG7:
-
Spastic paraplegia type 7
- SRH:
-
Second region of homology
- StAR:
-
Steroidogenic acute regulatory protein
- STUB1:
-
STIP1 homology and U-box containing protein 1
- TM1/2:
-
Trans-membrane region 1/2
- WFS:
-
Wolframin ER transmembrane glycoprotein
- YME1L:
-
YME1 like 1 ATPase
- Nrg1-III:
-
Neuregulin 1 type III
- HCLR:
-
HslU/ClpX, ClpABC-CTD, Lon, and R
References
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125:1241–1252. https://doi.org/10.1016/j.cell.2006.06.010
McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16:R551–R560. https://doi.org/10.1016/j.cub.2006.06.054
Bohovych ICS, Khalimonchuk O (2015) Mitochondrial protein quality control: the mechanisms guarding mitochondrial health. Antioxid Redox Signal 22(12):977–994. https://doi.org/10.1089/ars.2014.6199
Patron M, Sprenger H-G, Langer T (2018) m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res 28(3):296–306. https://doi.org/10.1038/cr.2018.17
Tsai MF, Phillips CB, Ranaghan M et al (2016) Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 5(pii):e15545. https://doi.org/10.7554/eLife.15545
Tsai CW, Wu Y, Pao PC, Phillips CB, Williams C, Miller C, Ranaghan M, Tsai MF (2017) Proteolytic control of the mitochondrial calcium uniporter complex. Proc Natl Acad Sci U S A 114(17):4388–4393. https://doi.org/10.1073/pnas.1702938114
Cuperfain AB, Zhang ZL, Kennedy JL, Gonçalves VF (2018) The complex interaction of mitochondrial genetics and mitochondrial pathways in psychiatric disease. Mol Neuropsychiatry 4(1):52–69. https://doi.org/10.1159/000488031
Kondadi AK, Wang S, Montagner S et al (2014) Loss of the m-AAA protease subunit AFG3L2 causes mitochondrial transport defects and tau hyperphosphorylation. EMBO J 33(9):1011–1026. https://doi.org/10.1002/embj.201387009
Bagli E, Zikou KA, Agnantis N, Kitsos G (2017) Mitochondrial membrane dynamics and inherited optic neuropathies. In Vivo 31(4):511–525. https://doi.org/10.21873/invivo.11090
Opalińska MJH (2018) AAA proteases: guardians of mitochondrial function and homeostasis. Cells 7(10):163. https://doi.org/10.3390/cells7100163
Levytskyy RM, Iryna B, Khalimonchuk O (2017) Metalloproteases of the inner mitochondrial membrane. Biochemistry 56(36):4737–4746. https://doi.org/10.1021/acs.biochem.7b00663
Richter U, Lahtinen T, Marttinen P, Suomi F, Battersby BJ (2015) Quality control of mitochondrial protein synthesis is required for membrane integrity and cell fitness. J Cell Biol 211(2):373–389. https://doi.org/10.1083/jcb.201504062
Charif M, Roubertie A, Salime S et al (2015) A novel mutation of AFG3L2 might cause dominant optic atrophy in patients with mild intellectual disability. Front Genet 6:311. https://doi.org/10.3389/fgene.2015.00311
Colavito D, Maritan V, Suppiej A, Del Giudice E, Mazzarolo M, Miotto S, Farina S, DalleCarbonare M et al (2017) Non-syndromic isolated dominant optic atrophy caused by the p.R468C mutation in the AFG3 like matrix AAA peptidase subunit 2 gene. Biomed Rep 7(5):451–454. https://doi.org/10.3892/br.2017.987
Sekine S, Youle RJ (2018) PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol 16(2). https://doi.org/10.1186/s12915-017-0470-7
Yang S, Xia C, Li S, Du L, Zhang L, Hu Y (2014) Mitochondrial dysfunction driven by the LRRK2-mediated pathway is associated with loss of Purkinje cells and motor coordination deficits in diabetic rat model. Cell Death Dis 5(5):e1217. https://doi.org/10.1038/cddis.2014.184
Teuling E, Bourgonje A, Veenje S, Thijssen K, de Boer J, van der Velde J, Swertz M, Nollen E (2011) Modifiers of mutant huntingtin aggregation: functional conservation of C. elegans-modifiers of polyglutamine aggregation. PLoS Curr 3(RRN1255). https://doi.org/10.1371/currents.RRN1255
Calandra CR, Buda G, Vishnopolska SA, Oliveri J, Olivieri FA, Pérez Millán MI, Biagioli G, Miquelini LA et al (2020) (2020) Spastic ataxia with eye-of-the-tiger-like sign in 4 siblings due to novel compound heterozygous AFG3L2 mutation. Parkinsonism Relat Disord 73:52–54. https://doi.org/10.1016/j.parkreldis.2020.03.020
Sacco T, Boda E, Hoxha E et al (2010) Mouse brain expression patterns of Spg7, Afg3l1, and Afg3l2 transcripts, encoding for the mitochondrial m-AAA protease. BMC Neurosci 11(55). https://doi.org/10.1186/1471-2202-11-55
Rawlings ND, Barrett A (2013) Introduction: aspartic and glutamic peptidases and their clans. Handbook of Proteolytic Enzymes, London
Khalimonchuk O, Jeong M, Watts T, Ferris E, Winge DR (2012) Selective Oma1 protease-mediated proteolysis of Cox1 subunit of cytochrome oxidase in assembly mutants. J Biol Chem 287(10):7289–7300. https://doi.org/10.1074/jbc.M111.313148
Magri S, Fracasso V, Plumari M et al (2018) Concurrent AFG3L2 and SPG7 mutations associated with syndromic parkinsonism and optic atrophy with aberrant OPA1 processing and mitochondrial network fragmentation. Hum Mutat 39(12):2060–2071. https://doi.org/10.1002/humu.23658
Pierson T, Adams D, Bonn F, Martinelli P, Cherukuri PF, Teer JK et al (2011) Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet 7(10):e1002325. https://doi.org/10.1371/journal.pgen.1002325
Banfi S, Bassi M, Andolfi G, Marchitiello A, Zanotta S, Ballabio A, Casari G, Franco B (1999) Identification and characterization of AFG3L2, a novel paraplegin-related gene. Genomics 59(1):51–58. https://doi.org/10.1006/geno.1999.5818
Cagnoli C, Mariotti C, Taroni F et al (2006) SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22-q11.2. Brain 129:235–242. https://doi.org/10.1093/brain/awh651
Mariotti C, Bella D, Di Donato S, Taroni F (2012) Spinocerebellar ataxia type 28. Handb Clin Neurol 103:575–579. https://doi.org/10.1016/B978-0-444-51892-7.00039-5
Koppen M, Bonn F, Ehses S, Langer T (2009) Autocatalytic processing of m-AAA protease subunits in mitochondria. Mol Biol Cell 20(19):4216–24. https://doi.org/10.1091/mbc.e09-03-0218
Mancuso G, Barth E, Crivello P, Rugarli EI (2012) Alternative splicing of Spg7, a gene involved in hereditary spastic paraplegia, encodes a variant of paraplegin targeted to the endoplasmic reticulum. PLoS One 7(5):e36337. https://doi.org/10.1371/journal.pone.0036337
Ding B, Martin D, Rampello AJ, Glynn SE (2018) Dissecting substrate specificities of the mitochondrial AFG3L2 protease. Biochemistry 57(28):4225–4235. https://doi.org/10.1021/acs.biochem.8b00565
Koppen M, Metodiev MD, Casari G, Rugarli EI, Langer T (2007) Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol Cell Biol 27:758–767. https://doi.org/10.1128/MCB.01470-06
Kress W, Weber-Ban E (2009) The alternating power stroke of a 6-cylinder AAA protease chaperone engine. Mol Cell 35(5):545–547. https://doi.org/10.1016/j.molcel.2009.08.013
Augustin S, Gerdes F, Lee S, Tsai FT, Langer T, Tatsuta T (2009) An intersubunit signaling network coordinates ATP hydrolysis by m-AAA proteases. Mol Cell 35(5):574–585. https://doi.org/10.1016/j.molcel.2009.07.018
Truscott KN, Lowth B, Strack PR, Dougan DA (2010) Diverse functions of mitochondrial AAA+ proteins: protein activation, disaggregation, and degradation. Biochem Cell Biol 88(1):97–108. https://doi.org/10.1139/o09-167
Glynn SE (2017) Multifunctional mitochondrial AAA proteases. Front Mol Biosci 4(34). https://doi.org/10.3389/fmolb.2017.00034
Ramelot TA, Yang Y, Sahu ID, Lee HW, Xiao R, Lorigan GA, Montelione GT, Kennedy MA (2013) NMR structure and MD simulations of the AAA protease intermembrane space domain indicates peripheral membrane localization within the hexaoligomer. FEBS Lett 587(21):3522–3528. https://doi.org/10.1016/j.febslet.2013.09.009
Puchades C, Ding B, Song A, Wiseman RL, Lander GC, Glynn SE (2019) Unique structural features of the mitochondrial AAA+ protease AFG3L2 reveal the molecular basis for activity in health and disease. Mol Cell 75(5):1073-1085.e6. https://doi.org/10.1016/j.molcel.2019.06.016
Kummer E, Ban N (2021) Mechanisms and regulation of protein synthesis in mitochondria. Nat Rev Mol Cell Biol 22(5):307–325. https://doi.org/10.1038/s41580-021-00332-2
Ng KY, Richter U, Jackson CB, Seneca S, Battersby BJ (2021) Translation of MT-ATP6 pathogenic variants reveals distinct regulatory consequences from the co-translational quality control of mitochondrial protein synthesis. Hum Mol Genet ddab314. https://doi.org/10.1093/hmg/ddab314
Bulteau AL, Bayot A (2011) Mitochondrial proteases and cancer. Biochim Biophys Acta 1807:595–601. https://doi.org/10.1016/j.bbabio.2010.12.011
Rugarli EI, Langer T (2012) Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31:1336–1349. https://doi.org/10.1038/emboj.2012.38
Konig T, Troder SE, Bakka K, Korwitz A, Richter-Dennerlein R, Lampe PA et al (2016) The m-AAA protease associated with neurodegeneration limits MCU activity in mitochondria. Mol Cell 64:148–162. https://doi.org/10.1016/j.molcel.2016.08.020
Levytskyy RM, Germany EM, Khalimonchuk O (2016) Mitochondrial quality control proteases in neuronal welfare. J Neuroimmune Pharmacol 11:629–644. https://doi.org/10.1007/s11481-016-9683-8
Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z et al (2017) Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543:443–446. https://doi.org/10.1038/nature21695
Dastidar SG, Pham MT, Mitchell MB, Yeom SG, Jordan S, Chang A, Sopher BL, La Spada AR (2020) 4E-BP1 protects neurons from misfolded protein stress and Parkinson’s disease toxicity by inducing the mitochondrial unfolded protein response. J Neurosci 40(45):8734–8745. https://doi.org/10.1523/jneurosci.0940-20.2020
Aldridge JE, Horibe T, Hoogenraad NJ (2007) Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One 2(9):e874. https://doi.org/10.1371/journal.pone.0000874
Bahat A, Perlberg S, Melamed-Book N, Lauria I, Langer T, Orly J (2014) StAR enhances transcription of genes encoding the mitochondrial proteases involved in its own degradation. Mol Endocrinol 28(2):208–24. https://doi.org/10.1210/me.2013-1275
Bahat A, Perlberg S, Melamed-Book N et al (2015) Transcriptional activation of LON Gene by a new form of mitochondrial stress: a role for the nuclear respiratory factor 2 in StAR overload response (SOR). Mol Cell Endocrinol 408:62–72. https://doi.org/10.1016/j.mce.2015.02.022
Pettus EH, Betarbet R, Cottrell B, Wallace DC, Madyastha V, Greenamyre JT (2000) Immunocytochemical characterization of the mitochondrially encoded ND1 subunit of complex I (NADH : ubiquinone oxidoreductase) in rat brain. J Neurochem 75(1):383–392. https://doi.org/10.1046/j.1471-4159.2000.0750383.x
Wang S, Jacquemyn J, Murru S, Martinelli P, Barth E, Langer T, Niessen CM, Rugarli EI (2016) The mitochondrial m-AAA protease prevents demyelination and hair greying. PLoS Genet 12(12):e1006463. https://doi.org/10.1371/journal.pgen
Almajan ER, Richter R, Paeger L et al (2012) AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival. J Clin Invest 122(11):4048–4058. https://doi.org/10.1172/JCI64604
Di Bella D, Lazzaro F, Brusco A et al (2010) Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet 42(4):313–321. https://doi.org/10.1038/ng.544
Murru S, Hess S, Barth E et al (2019) Astrocyte-specific deletion of the mitochondrial m-AAA protease reveals glial contribution to neurodegeneration. Glia 67(8):1526–1541. https://doi.org/10.1002/glia.23626
Maltecca F, Baseggio E, Consolato F et al (2015) Purkinje neuron Ca2+ influx reduction rescues ataxia in SCA28 model. J Clin Invest 125(1):263–274. https://doi.org/10.1172/JCI74770
Yamada K, Watanabe M (2002) Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int 77(2):94–108. https://doi.org/10.1046/j.0022-7722.2002.00021.x
Lin J, Kumari S, Kim C et al (2016) RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540:124–128. https://doi.org/10.1038/nature20558
Maltecca F, Aghaie A, Schroeder DG et al (2008) The mitochondrial protease AFG3L2 is essential for axonal development. J Neurosci 28(11):2827–2836. https://doi.org/10.1523/JNEUROSCI.4677-07.2008
Maltecca F, Casari G (2010) In vivo detection of oxidized proteins: a practical approach to tissue-derived mitochondria. Methods Mol Biol 648:257–67. https://doi.org/10.1007/978-1-60761-756-3_17. (Clifton, N.J.)
Zurita Rendón O, Silva Neiva L, Sasarman F, Shoubridge EA (2014) The arginine methyltransferase NDUFAF7 is essential for complex I assembly and early vertebrate embryogenesis. Hum Mol Genet 23(19):5159–5170. https://doi.org/10.1093/hmg/ddu239
Michailov GV, Sereda M, Brinkmann BG et al (2004) Axonal neuregulin-1 regulates myelin sheath thickness. Science 304(5671):700–703. https://doi.org/10.1126/science.1095862
de Waegh SM, Lee V, Brady ST (1992) Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68:451–463
Maltecca F, De Stefani D, Cassina L et al (2012) Respiratory dysfunction by AFG3L2 deficiency causes decreased mitochondrial calcium uptake via organellar network fragmentation. Hum Mol Genet 21(17):3858–3870. https://doi.org/10.1093/hmg/dds214
Mancini C, Hoxha E, Iommarini L, Brussino A, Richter U, Montarolo F, Cagnoli C, Parolisi R et al (2019) Mice harbouring a SCA28 patient mutation in AFG3L2 develop late-onset ataxia associated with enhanced mitochondrial proteotoxicity. Neurobiol Dis 124:14–28. https://doi.org/10.1016/j.nbd.2018.10.018
Koseler A, Ma F, Kilic ID, Morselli M, Kilic O, Pellegrini M (2020) Genome-wide DNA methylation profiling of blood from monozygotic twins discordant for myocardial infarction. In Vivo 34(1):11782. https://doi.org/10.21873/invivo
Hornig-Do HT, Tatsuta T, Buckermann A, Bust M, Kollberg G, Rötig A, Hellmich M, Nijtmans L et al (2012) Nonsense mutations in the COX1 subunit impair the stability of respiratory chain complexes rather than their assembly. EMBO J 31(5):1293–1307. https://doi.org/10.1038/emboj.2011.477
Cesnekova J, Rodinova M, Hansikova H, Zeman J, Stiburek L (2018) Loss of mitochondrial AAA proteases AFG3L2 and YME1L impairs mitochondrial structure and respiratory chain biogenesis. Int J Mol Sci 19(12):3930. https://doi.org/10.3390/ijms19123930
Li Y, Park J, Deng JH, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38(5–6):283–291. https://doi.org/10.1007/s10863-006-9052-z
Volonte D, Liu Z, Shiva S, Galbiati F (2016) Caveolin-1 controls mitochondrial function through regulation of m-AAA mitochondrial protease. Aging 8(10):2355–2369. https://doi.org/10.18632/aging. (Albany NY)
Bettegazzi B, Pelizzoni I, Salerno Scarzella F et al (2019) Upregulation of peroxiredoxin 3 protects Afg3l2-KO cortical neurons in vitro from oxidative stress: a paradigm for neuronal cell survival under neurodegenerative conditions. Oxid Med Cell Longev 2019(4721950). https://doi.org/10.1155/2019/4721950
Richter U, Ng KY, Suomi F et al (2019) Mitochondrial stress response triggered by defects in protein synthesis quality control. Life Sci Alliance 2(1):e201800219. https://doi.org/10.26508/lsa.201800219
Consolato F, Maltecca F, Tulli S, Sambri I, Casari G (2018) m-AAA and i-AAA complexes coordinate to regulate OMA1, the stress-activated supervisor of mitochondrial dynamics. J Cell Sci 131(7):jcs213546. https://doi.org/10.1242/jcs.213546
Pierson TM, Adams D, Bonn F, Martinelli P, Cherukuri PF, Teer JK, Hansen NF, Cruz P et al (2011) Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet 7(10):e1002325. https://doi.org/10.1371/journal.pgen.1002325
Merkwirth C, Dargazanli S, Tatsuta T et al (2008) Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22(4):476–488. https://doi.org/10.1101/gad.460708
Ehses S, Raschke I, Mancuso G et al (2009) Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187(7):1023–1036. https://doi.org/10.1083/jcb.200906084
MacVicar T, Langer T (2016) OPA1 processing in cell death and disease - the long and short of it. J Cell Sci 129(12):2297–2306. https://doi.org/10.1242/jcs.159186
Baker TA, Sauer RT (2006) ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem Sci 31:647–653. https://doi.org/10.1016/j.tibs.2006.10.006
Kasumu A, Bezprozvanny I (2012) Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum 11(3):630–639. https://doi.org/10.1007/s12311-010-0182-9
Ito M (2002) The molecular organization of cerebellar long-term depression. Nat Rev Neurosci 3(11):896–902. https://doi.org/10.1038/nrn962
Cesnekova J, Spacilova J, Hansikova H, Houstek J, Zeman J, Stiburek L (2016) LACE1 interacts with p53 and mediates its mitochondrial translocation and apoptosis. Oncotarget 7(30):47687–47698. https://doi.org/10.18632/oncotarget.9959
Logan CV, Szabadkai G, Sharpe JA et al (2014) Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 46(2):188–193. https://doi.org/10.1038/ng.2851
Liu JC, Liu J, Holmström KM, Menazza S, Parks RJ, Fergusson MM, Yu Z, Springer DA et al (2016) MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell Rep 16(6):1561–1573. https://doi.org/10.1016/j.celrep.2016.07.011
Paillard M, Csordas G, Szanda G et al (2017) Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep 18(10):2291–2300. https://doi.org/10.1016/j.celrep.2017.02.032
Raffaello A, De Stefani D, Sabbadin D et al (2013) The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 32(17):2362–2376. https://doi.org/10.1038/emboj.2013.157
Tulli S, Del Bondio A, Baderna V et al (2019) Pathogenic variants in the AFG3L2 proteolytic domain cause SCA28 through haploinsufficiency and proteostatic stress-driven OMA1 activation. J Med Genet 56(8):499–511. https://doi.org/10.1136/jmedgenet-2018-105766
Ashizawa T, Xia G (2016) Ataxia. Continuum (Minneap Minn). Mov Disord 22(4):1208–26. https://doi.org/10.1212/CON.0000000000000362
Manto M, Gandini J, Feil K, Strupp M (2020) Cerebellar ataxias: an update. Curr Opin Neurol 33(1):150–160. https://doi.org/10.1097/wco.0000000000000774
da Costa R (2010) Ataxia, paresis and paralysis.C.E. In:Ettinger SJ; Feldman EC. Textbook of Veterinary Internal Medicine. Elsevier, 2017
Löbbe AM, Kang J, Hilker R, Hackstein H, Müller U, Nolte D (2014) A novel missense mutation in AFG3L2 associated with late onset and slow progression of spinocerebellar ataxia type 28. J Mol Neurosci 52(4):493–496. https://doi.org/10.1007/s12031-013-0187-1
Coarelli G, Wirth T, Tranchant C, Koenig M, Durr A, Anheim M (2023) The inherited cerebellar ataxias: an update. J Neurol 270(1):208–222. https://doi.org/10.1007/s00415-022-11383-6
Kumari R, Kumar D, Brahmachari SK et al (2018) Paradigm for disease deconvolution in rare neurodegenerative disorders in Indian population: insights from studies in cerebellar ataxias. J Genet 97:589–609. https://doi.org/10.1007/s12041-018-0948-2
Edener U, Wollner J, Hehr U, Kohl Z, Schilling S, Kreuz F, Bauer P, Bernard V et al (2010) Early onset and slow progression of SCA28, a rare dominant ataxia in a large four-generation family with a novel AFG3L2 mutation. Eur J Hum Genet 18(8):965–968. https://doi.org/10.1038/ejhg.2010.40
Jia D, Tang B, Chen Z, Shi Y, Sun Z, Zhang L, Wang J, Xia K et al (2012) Spinocerebellar ataxia type 28 (SCA28) is an uncommon cause of dominant ataxia among Chinese kindreds. Int J Neurosci 122(10):560–562. https://doi.org/10.3109/00207454.2012.690796
Zühlke C, Mikat B, Timmann D, Wieczorek D, Gillessen-Kaesbach G, Bürk K (2015) Spinocerebellar ataxia 28: a novel AFG3L2 mutation in a German family with young onset, slow progression and saccadic slowing. Cerebellum Ataxias 2:19. https://doi.org/10.1186/s40673-015-0038-7
Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS et al (2012) Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13(4):378–385. https://doi.org/10.1038/embor.2012.14
Atorino L, Silvestri L, Koppen M, Cassina L, Ballabio A, Marconi R, Langer T, Casari G (2003) Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J Cell Biol 163(4):777–87. https://doi.org/10.1083/jcb.200304112
van Gassen KL, van der Heijden, de Bot ST, den Dunnen WF, van den Berg LH, Verschuuren-Bemelmans CC, Kremer HP, Veldink JH et al (2012) Genotype-phenotype correlations in spastic paraplegia type 7: a study in a large Dutch cohort. Brain 135:2994-3004https://doi.org/10.1093/brain/aws224
Chiang HL, Fuh JL, Tsai YS, Soong BW, Liao YC, Lee YC (2021) Expanding the phenotype of AFG3L2 mutations: late-onset autosomal recessive spinocerebellar ataxia. J Neurol Sci 428:117600. https://doi.org/10.1016/j.jns.2021.117600
Matilla-Dueñas A, Sanchez I, Corral-Juan M, Dávalos A, Alvarez R, Latorre P (2010) Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum 9(2):148–166. https://doi.org/10.1007/s12311-009-0144-2
Nasir J, Frima N, Pickard B, Malloy MP, Zhan L, Grünewald R (2006) Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov Disord 21(6):859–863. https://doi.org/10.1002/mds.20846
Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G (2009) Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci 29(29):9244–9254. https://doi.org/10.1523/JNEUROSCI.1532-09.2009
Myers KA, Warman Chardon J, Huang L, Boycott KM (2014) Deletion of AFG3L2 associated with spinocerebellar ataxia type 28 in the context of multiple genomic anomalies. Am J Med Genet A 164A(12):3209–3212. https://doi.org/10.1002/ajmg.a.36771
Tunc S, Dulovic-Mahlow M, Baumann H et al (2019) Spinocerebellar ataxia type 28-phenotypic and molecular characterization of a family with heterozygous and compound-heterozygous mutations in AFG3L2. Cerebellum 18(4):817–822. https://doi.org/10.1007/s12311-019-01036-2
Cagnoli C, Stevanin G, Brussino A et al (2010) Missense mutations in the AFG3L2 proteolytic domain account for ∼1.5% of European autosomal dominant cerebellar ataxias. Hum Mutat 31(10):1117–1124. https://doi.org/10.1002/humu.21342
Mancini C, Roncaglia P, Brussino A et al (2013) Genome-wide expression profiling and functional characterization of SCA28 lymphoblastoid cell lines reveal impairment in cell growth and activation of apoptotic pathways. BMC Med Genomics 6:22. https://doi.org/10.1186/1755-8794-6-22
Qu J, Wu CK, Zuzuárregui JR, Hohler AD (2015) A novel AFG3L2 mutation in a Somalian patient with spinocerebellar ataxia type 28. J Neurol Sci 358(1–2):530–531. https://doi.org/10.1016/j.jns.2015.10.003
Smets K, Deconinck T, Baets J, Sieben A, Martin JJ, Smouts I, Wang S, Taroni F et al (2014) Partial deletion of AFG3L2 causing spinocerebellar ataxia type 28. Neurol Genet 82(23):2092–2100. https://doi.org/10.1212/WNL.0000000000000491
Szpisjak L, Nemeth VL, Szepfalusi N et al (2017) Neurocognitive characterization of an SCA28 family caused by a novel AFG3L2 gene mutation. Cerebellum 16(5–6):979–985. https://doi.org/10.1007/s12311-017-0870-9
Gorman GS, Pfeffer G, Griffin H, Blakely EL, Kurzawa-Akanbi M, Gabriel J, Sitarz K, Roberts M et al (2015) Clonal expansion of secondary mitochondrial DNA deletions associated with spinocerebellar ataxia type 28. JAMA Neurol 72(1):106–111. https://doi.org/10.1001/jamaneurol.2014.1753
Roux T, Barbier M, Papin M, Davoine CS, Sayah S, Coarelli G, Charles P, SPATAX network et al (2020) Clinical, neuropathological, and genetic characterization of STUB1 variants in cerebellar ataxias: a frequent cause of predominant cognitive impairment. Genet Med. https://doi.org/10.1038/s41436-020-0899-x
Martinelli P, La Mattina V, Bernacchia A, Magnoni R, Cerri F, Cox G, Quattrini A, Casari G et al (2009) Genetic interaction between the m-AAA protease isoenzymes reveals novel roles in cerebellar degeneration. Hum Mol Genet 18(11):2001–2013. https://doi.org/10.1093/hmg/ddp124
Svenstrup K, Nielsen T, Aidt F et al (2017) SCA28: Novel mutation in the AFG3L2 proteolytic domain causes a mild cerebellar syndrome with selective type-1 muscle fiber atrophy. Cerebellum 16(1):62–67. https://doi.org/10.1007/s12311-016-0765-1
Almontashiri NA, Chen H, Mailloux RJ, Tatsuta T, Teng AC, Mahmoud AB, Ho T, Stewart NA et al (2014) SPG7 variant escapes phosphorylation-regulated processing by AFG3L2, elevates mitochondrial ROS, and is associated with multiple clinical phenotypes. Cell Rep 7(3):834–47. https://doi.org/10.1016/j.celrep.2014.03.051
Chen KB, Chen KC, Chang YL, Chang KL, Chang PC, Chang TT, Chen YC (2016) In silico investigation of traditional Chinese medicine for potential lead compounds as SPG7 inhibitors against coronary artery disease. Molecules 21(5):588. https://doi.org/10.3390/molecules21050588
Henke RM, Dastidatr RG, Shah A, Cadinu D, Yao X, Hooda J et al (2011) Hypoxia elicits broad and systematic changes in protein subcellular localization. Am J Physiol - Cell Physiol 301(4):C913–C928. https://doi.org/10.1152/ajpcell.00481.2010
Luciano M, Lopez L, de Moor MH, Harris SE, Davies G, Nutile T, Krueger RF, Esko T et al (2012) Longevity candidate genes and their association with personality traits in the elderly. Am J Med Genet B Neuropsychiatr Genet 159B(2):192–200. https://doi.org/10.1002/ajmg.b.32013
Lopez LM, Harris SE, Luciano M, Liewald D, Davies G, Gow AJ, Tenesa A, Payton A et al (2012) Evolutionary conserved longevity genes and human cognitive abilities in elderly cohorts. Eur J Hum Genet 20(3):341–347. https://doi.org/10.1038/ejhg.2011.201
Baderna V, Schultz J, Kearns LS et al (2020) A novel AFG3L2 mutation close to AAA domain leads to aberrant OMA1 and OPA1 processing in a family with optic atrophy. Acta Neuropathol Commun 8(1):93. https://doi.org/10.1186/s40478-020-00975-w
Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI et al (2007) OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell 18(9):3582–3590. https://doi.org/10.1091/mbc.e07-02-0164
Brussino A, Brusco A, Durr A, Mancini C, Adam MP, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (2011) Spinocerebellar ataxia type 28. GeneReviews® [Internet]: Seattle (WA): University of Washington, Seattle
Sainio MT, Aaltio J, Hyttinen V, Kortelainen M, Ojanen S, Paetau A, Tienari P, Ylikallio E et al (2022) Effectiveness of clinical exome sequencing in adult patients with difficult-to-diagnose neurological disorders. Acta Neurol Scand 145(1). https://doi.org/10.1111/ane.13522
TD B (1998) Hereditary Ataxia Overview.A.H. Adam MP, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A. GeneReviews® [Internet]: Seattle (WA): University of Washington, Seattle
Esmail S (2018) Cerebellar ataxia but normal neuroimaging: now what. J Med-Clin Res Rev 2(6):1–5
Catania A, Legati A, Peverelli L, Nanetti L, Marchet S, Zanetti N, Lamperti C, Ghezzi D (2019) Homozygous variant in OTX2 and possible genetic modifiers identified in a patient with combined pituitary hormone deficiency, ocular involvement, myopathy, ataxia, and mitochondrial impairment. Am J Med Genet A 179(5):827–831. https://doi.org/10.1002/ajmg.a.61092
Harvey NR, Voisin S, Lea RA, Yan X, Benton MC, Papadimitriou ID, Jacques M, Haupt LM et al (2020) Investigating the influence of mtDNA and nuclear encoded mitochondrial variants on high intensity interval training outcomes. Sci Rep 10(1):11089. https://doi.org/10.1038/s41598-020-67870-1
Dosi C, Galatolo D, Rubegni A, Doccini S, Pasquariello R, Nesti C, Sicca F, Barghigiani M et al (2020) Expanding the clinical and genetic heterogeneity of SPAX5. Ann Clin Transl Neurol 7(4):595–601. https://doi.org/10.1002/acn3.51024
Muona M, Berkovic S, Dibbens LM, Oliver KL, Maljevic S, Bayly MA et al (2015) A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat Genet 47(1):39–46. https://doi.org/10.1038/ng.3144
Canafoglia L, Franceschetti S, Gambardella A, Striano P, Giallonardo AT, Tinuper P, Di Bonaventura C, Michelucci R et al (2021) Progressive myoclonus epilepsies: diagnostic yield with next-generation sequencing in previously unsolved cases. Neurol Genet 7(6):e641. https://doi.org/10.1212/NXG.0000000000000641
Musova Z, Kaiserova M, Kriegova E, Fillerova R, Vasovcak P, Santava A, Mensikova K, Zumrova A et al (2014) A novel frameshift mutation in the AFG3L2 gene in a patient with spinocerebellar ataxia. Cerebellum 13(3):331–337. https://doi.org/10.1007/s12311-013-0538-z. (London, England)
Liu J, Wang X, Lu Y et al (2017) Pink1 interacts with α-synuclein and abrogates α-synuclein-induced neurotoxicity by activating autophagy. Cell Death Dis 8(9):e3056. https://doi.org/10.1038/cddis.2017.427
Li GB, Zhang H, Fu RQ, Hu XY, Liu L, Li YN, Liu YX, Liu X et al (2018) Mitochondrial fission and mitophagy depend on cofilin-mediated actin depolymerization activity at the mitochondrial fission site. Oncogene 37(11):1485–1502. https://doi.org/10.1038/s41388-017-0064-4
Thomas RE, Andrews LA, Burman JL, Lin WY, Pallanck LJ (2014) PINK1-Parkin pathway activity is regulated by degradation of PINK1 in the mitochondrial matrix. PLoS Genet 10(5):e1004279. https://doi.org/10.1371/journal.pgen.1004279
Steer EK, Dail MK, Chu CT (2015) Beyond mitophagy: cytosolic PINK1 as a messenger of mitochondrial health. Antioxid Redox Signal 22(12):1047–1059. https://doi.org/10.1089/ars.2014.6206
Dawson TM, Dawson V (2010) The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord 25:S32–S39. https://doi.org/10.1002/mds.22798
Berwick DC, Harvey K (2011) LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol 21(5):257–265. https://doi.org/10.1016/j.tcb.2011.01.001
Eskandrani A, AlHashem A, Ali ES et al (2017) Recessive AFG3L2 mutation causes progressive microcephaly, early onset seizures, spasticity, and basal ganglia involvement. Pediatr Neurol 71(24–28). https://doi.org/10.1016/j.pediatrneurol.2017.03.019
Grau T, Burbulla L, Engl G et al (2013) A novel heterozygous OPA3 mutation located in the mitochondrial target sequence results in altered steady-state levels and fragmented mitochondrial network. J Med Genet 50(12):848–858. https://doi.org/10.1136/jmedgenet-2013-101774
Reynier P, Amati-Bonneau P, Verny C et al (2004) OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract. J Med Genet 41(9):e110. https://doi.org/10.1136/jmg.2003.016576
Gerber S, Charif M, Chevrollier A et al (2017) Mutations in DNM1L, as in OPA1, result in dominant optic atrophy despite opposite effects on mitochondrial fusion and fission. Brain 140(10):2586–2596. https://doi.org/10.1093/brain/awx219
Rendtorff ND, Lodahi M, Boulahbel H et al (2011) Identification of p.A684V missense mutation in the WFS1 gene as a frequent cause of autosomal dominant optic atrophy and hearing impairment. Am J Med Genet A 155A(6):1298–1313. https://doi.org/10.1002/ajmg.a.33970
Charif M, Chevrollier A, Gueguen N, Bris C, Goudenège D, Desquiret-Dumas V, Leruez S, Colin E et al (2020) Mutations in the m-AAA proteases AFG3L2 and SPG7 are causing isolated dominant optic atrophy. Neurol Genet 6(3):e428. https://doi.org/10.1212/NXG.0000000000000428
Caporali L, Magri S, Legati A et al (2020) ATPase domain AFG3L2 mutations alter OPA1 processing and cause optic neuropathy. Ann Neurol 88(1):18–32. https://doi.org/10.1002/ana.25723
Mancini C, Orsi L, Guo Y, Li J, Chen Y, Wang F, Tian L, Liu X et al (2015) An atypical form of AOA2 with myoclonus associated with mutations in SETX and AFG3L2. BMC Med Genet 16(16). https://doi.org/10.1186/s12881-015-0159-0
Acknowledgements
The authors thank MAHE for infrastructure and software support.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This work was supported by the Department of Biotechnology, India, Ramalingaswami Fellowship grant number SAN No: 102.IFD/SAN/2549/2019–20 dated 29–10-2019 to SGD, Ramalingaswami Fellowship to PBL, and Manipal Academy of Higher Education Seed Ignition Grant to RGD.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RGD, SB, PBL, and SGD. The first draft of the manuscript was written by RGD, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. RGD and SGD conceptualized the review, and the literature survey was done by all the authors.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghosh Dastidar, R., Banerjee, S., Lal, P.B. et al. Multifaceted Roles of AFG3L2, a Mitochondrial ATPase in Relation to Neurological Disorders. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03768-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03768-z