Abstract
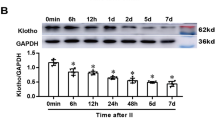
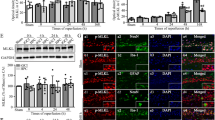
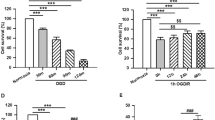
Our previous study has proved that the Klotho up-regulation participated in cerebral ischemic preconditioning (CIP)-induced brain ischemic tolerance. However, the exact neuroprotective mechanism of Klotho in CIP remains unclear. We explored the hypothesis that STAT4-mediated Klotho up-regulation contributes to the CIP-induced brain ischemic tolerance via inhibiting neuronal pyroptosis. Firstly, the expressions of pyroptosis-associated proteins (i.e., NLRP3, GSDMD, pro-caspase-1, and cleaved caspase-1) in hippocampal CA1 region were determined during the process of brain ischemic tolerance. We found the expression of pyroptosis-associated proteins was significantly up-regulated in the ischemic insult (II) group, and showed no significant changes in the CIP group. The expression level of each pyroptosis-associated proteins was lower in the CIP + II group than that in the II group. Inhibition of Klotho expression increased the expression of pyroptosis-associated proteins in the CIP + II group and blocked the CIP-induced brain ischemic tolerance. Injection of Klotho protein decreased the expression of pyroptosis-associated proteins in the II group, and protected neurons from ischemic injury. Secondly, the transcription factor STAT4 of Klotho was identified by bioinformatic analysis. Double luciferase reporter gene assay and chromatin immunoprecipitation assay showed STAT4 can bind to the site between nt − 881 and – 868 on the Klotho promoter region and positively regulates Klotho expression. Moreover, we found CIP significantly enhanced the expression of STAT4. Knockdown STAT4 suppressed Klotho up-regulation after CIP and blocked the CIP-induced brain ischemic tolerance. Collectively, it can be concluded that STAT4-mediated the up-regulation of Klotho contributed to the brain ischemic tolerance induced by CIP via inhibiting pyroptosis.








Similar content being viewed by others
Data Availability
The data used in the present study are available from the corresponding author on reasonable request.
Abbreviations
- AAV:
-
Adeno-associated virus
- BCCAs:
-
Bilateral common carotid arteries
- CIP:
-
Cerebral ischemic preconditioning
- ND:
-
Neuronal density
- DND:
-
Delayed neuronal death
- d:
-
Day
- GSDMD:
-
Gasdermin D
- h:
-
Hour
- II:
-
Ischemic insult
- i.c.v. :
-
Intracerebroventricular
- KL:
-
Klotho
- min:
-
Minute
- NC:
-
Negative control
- NLRP3:
-
NOD-like receptor family pyrin domain containing 3
- NS:
-
Statistically nonsignificant
- STAT:
-
Signal transducer and activator of transcription
- siRNA:
-
Small interfering RNA
References
Zhang S, Xu M, Liu ZJ, Feng J, Ma Y (2020) Neuropsychiatric issues after stroke: Clinical significance and therapeutic implications. World J Psychiatry 10(6):125–138. https://doi.org/10.5498/wjp.v10.i6.125
Johnson W, Onuma O, Owolabi M, Sachdev S (2016) Stroke: a global response is needed. Bull World Health Organ 94(9):634-634A. https://doi.org/10.2471/BLT.16.181636
Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7(6):437–448. https://doi.org/10.1038/nrn1927
Yin XH, Zhang QG, Miao B, Zhang GY (2005) Neuroprotective effects of preconditioning ischaemia on ischaemic brain injury through inhibition of mixed-lineage kinase 3 via NMDA receptor-mediated Akt1 activation. J Neurochem 93(4):1021–1029. https://doi.org/10.1111/j.1471-4159.2005.03096.x
Steiger HJ, Hanggi D (2007) Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir 149(1):1–10. https://doi.org/10.1007/s00701-006-1057-1. (Wien)
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51. https://doi.org/10.1038/36285
Abraham CR, Mullen PC, Tucker-Zhou T, Chen CD, Zeldich E (2016) Klotho Is a Neuroprotective and Cognition-Enhancing Protein. Vitam Horm 101:215–238. https://doi.org/10.1016/bs.vh.2016.02.004
Zhou HJ, Li H, Shi MQ, Mao XN, Liu DL, Chang YR, Gan YM, Kuang X et al (2017) Protective Effect of Klotho against Ischemic Brain Injury Is Associated with Inhibition of RIG-I/NF-kappaB Signaling. Front Pharmacol 8:950. https://doi.org/10.3389/fphar.2017.00950
Long FY, Shi MQ, Zhou HJ, Liu DL, Sang N, Du JR (2018) Klotho upregulation contributes to the neuroprotection of ligustilide against cerebral ischemic injury in mice. Eur J Pharmacol 820:198–205. https://doi.org/10.1016/j.ejphar.2017.12.019
Lee JB, Woo HG, Chang Y, Jin YM, Jo I, Kim J, Song TJ (2019) Plasma Klotho concentrations predict functional outcome at three months after acute ischemic stroke patients. Ann Med 51(3–4):262–269. https://doi.org/10.1080/07853890.2019.1617434
Karizmeh MS, Shabani M, Shabani M, Sardari M, Babaei JF, Nabavizadeh F, Sadr SS, Adeli S (2022) Preconditioning exercise reduces hippocampal neuronal damage via increasing Klotho expression in ischemic rats. Brain Res Bull 188:133–142. https://doi.org/10.1016/j.brainresbull.2022.07.022
Jin Z, Zhang Z, Ke J, Wang Y, Wu H (2021) Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxid Med Cell Longev 2021:1697070. https://doi.org/10.1155/2021/1697070
Herr DR, Yam TYA, Tan WSD, Koh SS, Wong WSF, Ong WY, Chayaburakul K (2020) Ultrastructural Characteristics of DHA-Induced Pyroptosis. Neuromolecular Med 22(2):293–303. https://doi.org/10.1007/s12017-019-08586-y
Gou X, Xu D, Li F, Hou K, Fang W, Li Y (2021) Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J Physiol Biochem 77(4):511–529. https://doi.org/10.1007/s13105-021-00817-w
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526(7575):666–671. https://doi.org/10.1038/nature15541
Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16(7):407–420. https://doi.org/10.1038/nri.2016.58
Wang S, Yuan YH, Chen NH, Wang HB (2019) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol 67:458–464. https://doi.org/10.1016/j.intimp.2018.12.019
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610):153–158. https://doi.org/10.1038/nature18629
de Vasconcelos NM, Lamkanfi M (2020) Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb Perspect Biol 12(5). https://doi.org/10.1101/cshperspect.a036392
An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X, Li L, Tang M (2019) Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci 232:116599. https://doi.org/10.1016/j.lfs.2019.116599
Liu J, He J, Huang Y, Ge L, Xiao H, Zeng L, Jiang Z, Lu M et al (2021) Hypoxia-preconditioned mesenchymal stem cells attenuate microglial pyroptosis after intracerebral hemorrhage. Ann Transl Med 9(17):1362. https://doi.org/10.21037/atm-21-2590
Liu H, Zhao Z, Wu T, Zhang Q, Lu F, Gu J, Jiang T, Xue J (2021) Inhibition of autophagy-dependent pyroptosis attenuates cerebral ischaemia/reperfusion injury. J Cell Mol Med 25(11):5060–5069. https://doi.org/10.1111/jcmm.16483
Zhong Y, Li YP, Yin YQ, Hu BL, Gao H (2020) Dexmedetomidine inhibits pyroptosis by down-regulating miR-29b in myocardial ischemia reperfusion injury in rats. Int Immunopharmacol 86:106768. https://doi.org/10.1016/j.intimp.2020.106768
She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, Deng C, Liu X (2019) Neuroprotective effect of glycosides in Buyang Huanwu Decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol 242:112051. https://doi.org/10.1016/j.jep.2019.112051
Nie C, Ding X, Rong A, Zheng M, Li Z, Pan S, Yang W (2021) Hydrogen gas inhalation alleviates myocardial ischemia-reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyroptosis in rats. Life Sci 272:119248. https://doi.org/10.1016/j.lfs.2021.119248
Cao X, Wang Y, Gao L (2021) CHRFAM7A Overexpression Attenuates Cerebral Ischemia-Reperfusion Injury via Inhibiting Microglia Pyroptosis Mediated by the NLRP3/Caspase-1 pathway. Inflammation 44(3):1023–1034. https://doi.org/10.1007/s10753-020-01398-4
Sun R, Peng M, Xu P, Huang F, Xie Y, Li J, Hong Y, Guo H et al (2020) Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation 17(1):330. https://doi.org/10.1186/s12974-020-01988-x
Chang Y, Zhu J, Wang D, Li H, He Y, Liu K, Wang X, Peng Y et al (2020) NLRP3 inflammasome-mediated microglial pyroptosis is critically involved in the development of post-cardiac arrest brain injury. J Neuroinflammation 17(1):219. https://doi.org/10.1186/s12974-020-01879-1
Zhu X, Li S, Lin Q, Shao X, Wu J, Zhang W, Cai H, Zhou W et al (2021) alphaKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res 167:105531. https://doi.org/10.1016/j.phrs.2021.105531
Zhang LY, Liu XY, Su AC, Hu YY, Zhang JG, Xian XH, Li WB, Zhang M (2022) Klotho Upregulation via PPARgamma Contributes to the Induction of Brain Ischemic Tolerance by Cerebral Ischemic Preconditioning in Rats. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-022-01255-y
Darnell JE Jr (1997) STATs and gene regulation. Science 277(5332):1630–1635. https://doi.org/10.1126/science.277.5332.1630
Bromberg J, Darnell J (2000) The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19(21):2468–2473. https://doi.org/10.1038/sj.onc.1203476
Mehrpouya-Bahrami P, Moriarty AK, De Melo P, Keeter WC, Alakhras NS, Nelson AS, Hoover M, Barrios MS et al (2021) STAT4 is expressed in neutrophils and promotes antimicrobial immunity. JCI Insight 6(14). https://doi.org/10.1172/jci.insight.141326
Jiang Y, Xin X, Pan X, Zhang A, Zhang Z, Li J, Yuan X (2020) STAT4 targets KISS1 to promote the apoptosis of ovarian granulosa cells. J Ovarian Res 13(1):135. https://doi.org/10.1186/s13048-020-00741-5
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10(3):267–272. https://doi.org/10.1161/01.str.10.3.267
Zhang M, Gong J, Wang J, Li W (2017) p38 MAPK Participates in the Mediation ofGLT-1 Up-regulation During the Induction of Brain Ischemic Tolerance by Cerebral Ischemic Preconditioning. https://doi.org/10.1007/s12035-015-9652-x
Liu Y-X, Zhang M, Liu L-Z, Cui X, Hu Y-Y, Li W-B (2012) The role of glutamate transporter-1a in the induction of brain ischemic tolerance in rats. Glia 60(1):112–124. https://doi.org/10.1002/glia.21252
Su AC, Zhang LY, Zhang JG, Hu YY, Liu XY, Li SC, Xian XH, Li WB et al (2022) The Regulation of Autophagy by p38 MAPK-PPARgamma Signaling During the Brain Ischemic Tolerance Induced by Cerebral Ischemic Preconditioning. DNA Cell Biol 41(9):838–849. https://doi.org/10.1089/dna.2022.0087
Zhang M, Li WB, Geng JX, Li QJ, Sun XC, Xian XH, Qi J, Li SQ (2007) The upregulation of glial glutamate transporter-1 participates in the induction of brain ischemic tolerance in rats. J Cereb Blood Flow Metab 27(7):1352–1368. https://doi.org/10.1038/sj.jcbfm.9600441
Dong Z, Pan K, Pan J, Peng Q, Wang Y (2018) The Possibility and Molecular Mechanisms of Cell Pyroptosis After Cerebral Ischemia. Neurosci Bull 34(6):1131–1136. https://doi.org/10.1007/s12264-018-0294-7
Ismael S, Zhao L, Nasoohi S, Ishrat T (2018) Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 8(1):5971. https://doi.org/10.1038/s41598-018-24350-x
Alishahi M, Farzaneh M, Ghaedrahmati F, Nejabatdoust A, Sarkaki A, Khoshnam SE (2019) NLRP3 inflammasome in ischemic stroke: As possible therapeutic target. Int J Stroke 14(6):574–591. https://doi.org/10.1177/1747493019841242
Lorenz G, Darisipudi MN, Anders HJ (2014) Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29(1):41–48. https://doi.org/10.1093/ndt/gft332
Wang K, Ru J, Zhang H, Chen J, Lin X, Lin Z, Wen M, Huang L et al (2020) Melatonin Enhances the Therapeutic Effect of Plasma Exosomes Against Cerebral Ischemia-Induced Pyroptosis Through the TLR4/NF-kappaB Pathway. Front Neurosci 14:848. https://doi.org/10.3389/fnins.2020.00848
Poh L, Kang SW, Baik SH, Ng GYQ, She DT, Balaganapathy P, Dheen ST, Magnus T et al (2019) Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun 75:34–47. https://doi.org/10.1016/j.bbi.2018.09.001
Li J, Hao JH, Yao D, Li R, Li XF, Yu ZY, Luo X, Liu XH et al (2020) Caspase-1 inhibition prevents neuronal death by targeting the canonical inflammasome pathway of pyroptosis in a murine model of cerebral ischemia. CNS Neurosci Ther 26(9):925–939. https://doi.org/10.1111/cns.13384
Lv Y, Sun B, Lu XX, Liu YL, Li M, Xu LX, Feng CX, Ding X et al (2020) The role of microglia mediated pyroptosis in neonatal hypoxic-ischemic brain damage. Biochem Biophys Res Commun 521(4):933–938. https://doi.org/10.1016/j.bbrc.2019.11.003
Yang KL, Li WH, Liu YJ, Wei YJ, Ren YK, Mai CD, Zhang SY, Zuo Y et al (2022) Hydrogen Sulfide Attenuates Neuroinflammation by Inhibiting the NLRP3/Caspase-1/GSDMD Pathway in Retina or Brain Neuron following Rat Ischemia/Reperfusion. Brain Sci 12(9). https://doi.org/10.3390/brainsci12091245
Lu D, Hu M, Zhang B, Lin Y, Zhu Q, Men X, Lu Z, Cai W (2021) Temporal and Spatial Dynamics of Inflammasome Activation After Ischemic Stroke. Front Neurol 12:621555. https://doi.org/10.3389/fneur.2021.621555
Kang X, Jiang L, Chen X, Wang X, Gu S, Wang J, Zhu Y, Xie X et al (2021) Exosomes derived from hypoxic bone marrow mesenchymal stem cells rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis. Exp Cell Res 405(1):112635. https://doi.org/10.1016/j.yexcr.2021.112635
Wang L, Ren W, Wu Q, Liu T, Wei Y, Ding J, Zhou C, Xu H et al (2022) NLRP3 Inflammasome Activation: A Therapeutic Target for Cerebral Ischemia-Reperfusion Injury. Front Mol Neurosci 15:847440. https://doi.org/10.3389/fnmol.2022.847440
Xiang T, Luo X, Ye L, Huang H, Wu Y (2022) Klotho alleviates NLRP3 inflammasome-mediated neuroinflammation in a temporal lobe epilepsy rat model by activating the Nrf2 signaling pathway. Epilepsy Behav 128:108509. https://doi.org/10.1016/j.yebeh.2021.108509
Zhua L, Steina LR, Kima D, Hoa K, Yua G-Q, Zhana L, Larssonb TE, Muckea L (2018) Klotho controls the brain–immune system interface in the choroid plexus. Proc Natl Acad Sci USA 115(48):E11388–E11396. https://doi.org/10.1073/pnas.1808609115
Li Y, Liu Y, Wang K, Huang Y, Han W, Xiong J, Yang K, Liu M et al (2020) Klotho is regulated by transcription factor Sp1 in renal tubular epithelial cells. BMC Mol Cell Biol 21(1):45. https://doi.org/10.1186/s12860-020-00292-z
Li Y, Liu Y, Huang Y, Yang K, Xiao T, Xiong J, Wang K, Liu C et al (2020) IRF-1 promotes renal fibrosis by downregulation of Klotho. FASEB J 34(3):4415–4429. https://doi.org/10.1096/fj.201902446R
Li M, Liu Y, Fu Y, Gong R, Xia H, Huang X, Wu Y (2021) Interleukin-35 inhibits lipopolysaccharide-induced endothelial cell activation by downregulating inflammation and apoptosis. Exp Cell Res 407(2):112784. https://doi.org/10.1016/j.yexcr.2021.112784
He M, Li M, Guo Z (2022) STAT4 regulates cardiomyocyte apoptosis in rat models of diabetic cardiomyopathy. Acta Histochem 124(4):151872. https://doi.org/10.1016/j.acthis.2022.151872
Acknowledgements
We thank Dr. Chenguang Zhao for her linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Natural Science Foundation of Hebei Province (Nos. H2021206160 and H2022206579) and the National Natural Science Foundation of China (No. 81971228).
Author information
Authors and Affiliations
Contributions
Xi-Yun Liu and Ling-Yan Zhang performed experiments. Xi-Yun Liu, Shi-chao Li, and Xiao-Yu Wang analyzed data and drafted the manuscript. Yu-Yan Hu and Xiao-Hui Xian drafted the figures. Min Zhang, Jing-Ge Zhang, and Wen-Bin Li designed the study and contributed to editing the final draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animals in this study were approved by the Laboratory Animal Ethical and Welfare Committee of Hebei Medical University, China (Approval No. IACUC-Hebmu–2020013).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no potential conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, XY., Zhang, LY., Wang, XY. et al. STAT4-Mediated Klotho Up-Regulation Contributes to the Brain Ischemic Tolerance by Cerebral Ischemic Preconditioning via Inhibiting Neuronal Pyroptosis. Mol Neurobiol 61, 2336–2356 (2024). https://doi.org/10.1007/s12035-023-03703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03703-2