Abstract
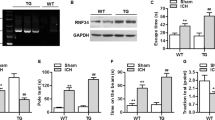
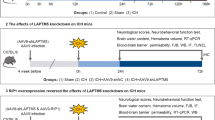
The deubiquitylase OTU domain-containing ubiquitin aldehyde-binding protein 1 (OTUB1) has been implicated in the pathogenesis of various human diseases. However, the molecular mechanism by which OTUB1 participates in the pathogenesis of intracerebral hemorrhage (ICH) remains elusive. In the present study, we established an autologous whole blood fusion-induced ICH model in C57BL/6 J mice. We showed that the upregulation of OTUB1 contributes to the attenuation of Nuclear factor kappa B (NF-κB) and its downstream apoptotic signaling after ICH. OTUB1 directly associates with NF-κB precursors p105 and p100 after ICH, leading to attenuated polyubiquitylation of p105 and p100. Moreover, we revealed that NF-κB signaling was modestly activated both in ICH tissues and hemin-exposed HT-22 neuronal cells, accompanied with the activation of NF-κB downstream pro-apoptotic signaling. Notably, overexpression of OTUB1 strongly inhibited hemin-induced NF-κB activation, whereas interference of OTUB1 led to the opposite effect. Finally, we revealed that lentiviral transduction of OTUB1 markedly ameliorated hemin-induced apoptotic signaling and HT-22 neuronal death. Collectively, these findings suggest that the upregulation of OTUB1 serves as a neuroprotective mechanism in antagonizing neuroinflammation-induced NF-κB signaling and neuronal death, shed new light on manipulating intracellular deubiquitylating pathways as novel interventive approaches against ICH-induced secondary neuronal damage and death.







Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
References
Fernando SM, Qureshi D, Talarico R, Tanuseputro P, Dowlatshahi D, Sood MM, Smith EE, Hill MD et al (2021) Intracerebral hemorrhage incidence, mortality, and association with oral anticoagulation use: A population study. Stroke 52:1673–1681. https://doi.org/10.1161/STROKEAHA.120.032550
Sheth KN (2022) Spontaneous intracerebral hemorrhage. N Engl J Med 387:1589–1596. https://doi.org/10.1056/NEJMra2201449
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63. https://doi.org/10.1016/S1474-4422(05)70283-0
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, Che F, Chen X et al (2019) Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol 178:101610. https://doi.org/10.1016/j.pneurobio.2019.03.003
Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, Simard JM, Sheth KN (2015) Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol 11:111–122. https://doi.org/10.1038/nrneurol.2014.264
Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J (1999) Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke 30:2472–7. https://doi.org/10.1161/01.str.30.11.2472. (discussion 2477-8)
Wang YX, Yan A, Ma ZH, Wang Z, Zhang B, Ping JL, Zhu JS, Zhou Y et al (2011) Nuclear factor-kappaB and apoptosis in patients with intracerebral hemorrhage. J Clin Neurosci 18:1392–1395. https://doi.org/10.1016/j.jocn.2010.11.039
Liu Q, Zhang Y (2019) PRDX1 enhances cerebral ischemia-reperfusion injury through activation of TLR4-regulated inflammation and apoptosis. Biochem Biophys Res Commun 519:453–461. https://doi.org/10.1016/j.bbrc.2019.08.077
Xi Z, Hu X, Chen X, Yang Y, Ren J, Wang B, Zhong Z, Sun Y et al (2019) Protocatechuic acid exerts protective effects via suppression of the P38/JNK- NF-kappaB signalling pathway in an experimental mouse model of intracerebral haemorrhage. Eur J Pharmacol 854:128–138. https://doi.org/10.1016/j.ejphar.2019.03.008
Zeng J, Zheng S, Chen Y, Qu Y, Xie J, Hong E, Lv H, Ding R et al (2021) Puerarin attenuates intracerebral hemorrhage-induced early brain injury possibly by PI3K/Akt signal activation-mediated suppression of NF-kappaB pathway. J Cell Mol Med 25:7809–7824. https://doi.org/10.1111/jcmm.16679
Dai Y, Zhang H, Zhang J, Yan M (2018) Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chem Biol Interact 284:32–40. https://doi.org/10.1016/j.cbi.2018.02.017
Napolitano M, Zei D, Centonze D, Palermo R, Bernardi G, Vacca A, Calabresi P, Gulino A (2008) NF-kB/NOS cross-talk induced by mitochondrial complex II inhibition: implications for Huntington’s disease. Neurosci Lett 434:241–246. https://doi.org/10.1016/j.neulet.2007.09.056
Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S (1997) Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 198:35–49. https://doi.org/10.1016/s0171-2985(97)80025-3
Wu ZH, Shi Y, Tibbetts RS, Miyamoto S (2006) Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311:1141–1146. https://doi.org/10.1126/science.1121513
Christian F, Smith EL and Carmody RJ (2016) The regulation of NF-kappaB Subunits by phosphorylation. Cells 5. https://doi.org/10.3390/cells5010012
Jamal A, Husein A, Bihari C, Kumar V (2020) Ubiquitin ligase TRUSS augments the expression of interleukin-10 via proteasomal processing of NF-kappaB1/p105 to NF-kappaB/p50. Cell Signal 75:109766. https://doi.org/10.1016/j.cellsig.2020.109766
Park MH and Hong JT (2016) Roles of NF-kappaB in cancer and inflammatory diseases and their therapeutic approaches. Cells 5. https://doi.org/10.3390/cells5020015
Sun XX, Challagundla KB, Dai MS (2012) Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain 1. EMBO J 31:576–592. https://doi.org/10.1038/emboj.2011.434
Goncharov T, Niessen K, de Almagro MC, Izrael-Tomasevic A, Fedorova AV, Varfolomeev E, Arnott D, Deshayes K et al (2013) OTUB1 modulates c-IAP1 stability to regulate signalling pathways. EMBO J 32:1103–1114. https://doi.org/10.1038/emboj.2013.62
Liu T, Jiang L, Tavana O, Gu W (2019) The Deubiquitylase OTUB1 mediates Ferroptosis via stabilization of SLC7A11. Cancer Res 79:1913–1924. https://doi.org/10.1158/0008-5472.CAN-18-3037
Herhaus L, Al-Salihi M, Macartney T, Weidlich S, Sapkota GP (2013) OTUB1 enhances TGFbeta signalling by inhibiting the ubiquitylation and degradation of active SMAD2/3. Nat Commun 4:2519. https://doi.org/10.1038/ncomms3519
Liao Y, Yang M, Wang K, Wang Y, Zhong B, Jiang N (2022) Deubiquitinating enzyme OTUB1 in immunity and cancer: Good player or bad actor? Cancer Lett 526:248–258. https://doi.org/10.1016/j.canlet.2021.12.002
Koschel J, Nishanth G, Just S, Harit K, Kroger A, Deckert M, Naumann M, Schluter D (2021) OTUB1 prevents lethal hepatocyte necroptosis through stabilization of c-IAP1 during murine liver inflammation. Cell Death Differ 28:2257–2275. https://doi.org/10.1038/s41418-021-00752-9
Zhang HH, Li C, Ren JW, Liu L, Du XH, Gao J, Liu T, Li SZ (2021) OTUB1 facilitates bladder cancer progression by stabilizing ATF6 in response to endoplasmic reticulum stress. Cancer Sci 112:2199–2209. https://doi.org/10.1111/cas.14876
Wang P, Joberty G, Buist A, Vanoosthuyse A, Stancu IC, Vasconcelos B, Pierrot N, Faelth-Savitski M et al (2017) Tau interactome mapping based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol 133:731–749. https://doi.org/10.1007/s00401-016-1663-9
Xie L, Li A, Shen J, Cao M, Ning X, Yuan D, Ji Y, Wang H et al (2016) OTUB1 attenuates neuronal apoptosis after intracerebral hemorrhage. Mol Cell Biochem 422:171–180. https://doi.org/10.1007/s11010-016-2817-8
Wu H, Wang J, Cao M, Liang J, Wu D, Gu X, Ke K (2020) Effects of homocysteine-induced endoplasmic reticulum protein on endoplasmic reticulum stress, autophagy, and neuronal apoptosis following intracerebral hemorrhage. IBRO Rep 9:207–217. https://doi.org/10.1016/j.ibror.2020.08.004
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc 2016. https://doi.org/10.1101/pdb.prot087205
Li Y, Yang JY, Xie X, Jie Z, Zhang L, Shi J, Lin D, Gu M et al (2019) Preventing abnormal NF-kappaB activation and autoimmunity by Otub1-mediated p100 stabilization. Cell Res 29:474–485. https://doi.org/10.1038/s41422-019-0174-3
Zhang Z, Liu Y, Huang Q, Su Y, Zhang Y, Wang G, Li F (2014) NF-kappaB activation and cell death after intracerebral hemorrhage in patients. Neurol Sci 35:1097–1102. https://doi.org/10.1007/s10072-014-1657-0
Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ (2020) Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 22:29–46. https://doi.org/10.5853/jos.2019.02236
Liu ZC, Meng LQ, Song JH, Gao J (2018) Dynamic protein expression of NF-kappaB following rat intracerebral hemorrhage and its association with apoptosis. Exp Ther Med 16:3903–3908. https://doi.org/10.3892/etm.2018.6715
Lan X, Han X, Liu X, Wang J (2019) Inflammatory responses after intracerebral hemorrhage: From cellular function to therapeutic targets. J Cereb Blood Flow Metab 39:184–186. https://doi.org/10.1177/0271678X18805675
Liu DL, Zhao LX, Zhang S, Du JR (2016) Peroxiredoxin 1-mediated activation of TLR4/NF-kappaB pathway contributes to neuroinflammatory injury in intracerebral hemorrhage. Int Immunopharmacol 41:82–89. https://doi.org/10.1016/j.intimp.2016.10.025
Mulero MC, Huxford T, Ghosh G (2019) NF-kappaB, IkappaB, and IKK: Integral Components of Immune System Signaling. Adv Exp Med Biol 1172:207–226. https://doi.org/10.1007/978-981-13-9367-9_10
Kravtsova-Ivantsiv Y, Ciechanover A (2015) The ubiquitin-proteasome system and activation of NF-kappaB: involvement of the ubiquitin ligase KPC1 in p105 processing and tumor suppression. Mol Cell Oncol 2:e1054552. https://doi.org/10.1080/23723556.2015.1054552
Visekruna A, Joeris T, Seidel D, Kroesen A, Loddenkemper C, Zeitz M, Kaufmann SH, Schmidt-Ullrich R et al (2006) Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. J Clin Invest 116:3195–3203. https://doi.org/10.1172/JCI28804
Funding
This work was supported by the National Natural Science Foundation of China (81901195; 81873742).
Author information
Authors and Affiliations
Contributions
Conceptualization, J. S., R. C. and K. K.; methodology, J. S., X. X. and H. Y.; formal analysis, J. S. and X. X.; investigation, J. S., X. X. and K. K.; resources, J. S., H. Y., Y. S. and J. W.; data curation, J. S., X. X. and K. K.; writing—original draft preparation, J. S., X. X. and H. Y.; writing—review and editing, R. C. and K. K.; visualization, J. S., X. X. and Y. S.; supervision, R. C. and K. K.; project administration, R. C. and K. K.; funding acquisition, R. C. and K. K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental procedures were conducted in accordance with the National Institutes of Health (NIH) laboratory animal care and use guidelines, and have been approved by the Animal Ethics Committee of Nantong University (ethics number: S20180816-012).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing Interests
The authors declare that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Xue, X., Yuan, H. et al. Deubiquitylating Enzyme OTUB1 Facilitates Neuronal Survival After Intracerebral Hemorrhage Via Inhibiting NF-κB-triggered Apoptotic Cascades. Mol Neurobiol 61, 1726–1736 (2024). https://doi.org/10.1007/s12035-023-03676-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03676-2