Abstract
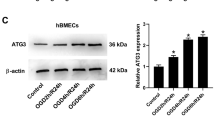
This work mainly aimed to explore the role and mechanism of advanced glycation end-products (AGEs) in inducing cerebrovascular endothelial cell pyroptosis under oxygen glucose deprivation (OGD) condition. The mouse cerebral microvascular endothelial cells (BMECs and bEnd.3) were used as the objects to construct the OGD model in vitro. Then, cells were pretreated with AGE-modified human serum albumin (AGE-HSA). Thereafter, CCK-8 assay was conducted to detect cell viability, and flow cytometry (FCM) was performed to measure cell pyroptosis level. Meanwhile, the expression of inflammatory factors was detected by enzyme-linked immunosorbent assay (ELISA). The expression of HIF-α, NLRP3, and RAGE was detected by fluorescence staining. The opening status of cell membrane pore was observed under the electron microscope, and the expression levels of FL-GSDMD, NT-GSDMD, and caspase-1 were measured through Western Blot (WB) assay. Moreover, bEnd.3 cells were treated with siRAN-silenced NLRP3 and HIF-α inhibitor, so as to observe the effect of AGEs on cell pyroptosis level. In the mouse model, the middle cerebral artery occlusion (MCAO) model was constructed by the suture-occluded method. After intraperitoneal injection of AGEs, the pathological changes in mouse brain tissues were detected; the expression levels of NLRP3, ZO-1, and CD31 were determined by histochemical staining, and the levels of inflammatory factors and pyroptosis-related proteins were also detected. Under OGD condition, AGEs induced the pyroptosis of bEnd.3 cells, and the cell pyroptosis rate increased, higher than that of the OGD group. Meanwhile, the levels of inflammatory factors were up-regulated; the expression of HIF-α, NLRP3, and RAGE in cells increased; and the levels of NT-GSDMD and caspase-1 were markedly higher than those of the control and OGD groups. siRNA-NLRP3 or HIF-α inhibitor treatment suppressed pyroptosis and reduced the inflammatory factor levels. In mouse experiments, AGE injection aggravated brain injury in the MCAO mouse model, decreased the expression of ZO-1 and CD31, and elevated the levels of NLRP3 and inflammatory factors. Under cerebral ischemia condition, AGEs can induce endothelial cell pyroptosis via HIF-α-RAGE-NLRP3, thereby further aggravating brain injury.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Nasution I, Sjahrir H, Ilyas S et al (2021) The effect of Ophiocephalus striatus sp. extract on nitric oxide in ischemic stroke model[J]. Open Access Maced J Med Sci 9(3):68–74
Acharya A, Yogi P, Singh P et al (2021) Herlyn Werner Wunderlich syndrome presenting with ischemic stroke due to suspected paroxysmal nocturnal hemoglobinuria: a case report[J]. JNMA; J Nepal Med Assoc 59(234):113–129
Wang J, Zhao J, Li S (2021) Research progress on the therapeutic effect of olfactory ensheathing cell transplantation on ischemic stroke[J]. J Neurorestoratology 9(2):83–93
Jiang J, Tan C, Zhou W, Peng W, Zhou X, Du J, Wang H, Mo L, Liu X, Chen L (2021) Plasma C-reactive protein level and outcome of acute ischemic stroke patients treated by intravenous thrombolysis: a systematic review and meta-analysis. Eur Neurol 84(3):145–150
Ikeda T, Maruyama K, Ito N et al (2012) Serum pentosidine, an advanced glycation end product, indicates poor outcomes after acute ischemic stroke.[J]. J Stroke Cerebrovasc Dis 21(5):386–390
Tang SC, Wang YC, Li YI et al (2013) Functional role of soluble receptor for advanced glycation end products in stroke.[J]. Arterioscler Thromb Vasc Biol 33(3):585–594
Ikeda, Maruyama, Ito et al (2012) Serum pentosidine, an advanced glycation end product, indicates poor outcomes after acute ischemic stroke[J]. J stroke cerebrovasc dis: off j Natl Stroke Assoc 21(5):386–390
Cheng YL, Park JS, Manzanero S et al (2014) Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke.[J]. Neurobiol Dis 62:286–295
Bala K, Gohil NK (2012) Interaction of glycated protein and DFO mimicked hypoxia in cellular responses of HUVECs[J]. Mol BioSyst 8(10):2657–2663
Xiaojun F, Xu Z, Qi W et al (2018) Xijiao Dihuang decoction alleviates ischemic brain injury in MCAO rats by regulating inflammation, neurogenesis, and angiogenesis[J]. Evid Based Complement Altern Med 2018:1–12
Wang HL, Liu FL, Li RQ et al (2021) Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation[J]. Neural Regen Res 16(6):1011
Guo H, Zhu L, Tang P et al (2021) Carthamin yellow improves cerebral ischemiareperfusion injury by attenuating inflammation and ferroptosis in rats[J]. Int J Mol Med 47(4):1311–1321
Mehta K, Bhagwat DP, Devraj et al (2022) Vitex negundo protects against cerebral ischemia–reperfusion injury in mouse via attenuating behavioral deficits and oxidative damage[J]. Psychopharmacol 239(2):573–587
Lei WA, Xx A, Xu ZA et al (2020) Sodium tanshinone IIA sulfonate protects against cerebral ischemia–reperfusion injury by inhibiting autophagy and inflammation - ScienceDirect[J]. Neurosci 441:46–57
Yang Y, Hu F, Yang G et al (2020) Lack of sphingomyelin synthase 2 reduces cerebral ischemia/reperfusion injury by inhibiting microglial inflammation in mice[J]. Exp Ther Med 20(6):176–182
Vajtr D, Benada O, Kukacka J et al (2009) Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response.[J]. Physiol Res 58(2):263–268
Zipser BD, Johanson CE, Gonzalez L et al (2007) Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease[J]. Neurobiol Aging 28(7):977–986
Oikonomou E, Tsioufis C, Tousoulis D (2021) Diabetes mellitus: a primary metabolic disturbance. Metabolomics underlying vascular responses to stress and ischemia?[J]. Clin Sci 135(3):589–591
Wang M, Li Y, Zhang R et al (2021) Adiponectin-transfected endothelial progenitor cells have protective effects after 2-hour middle-cerebral artery occlusion in rats with type 2 diabetes mellitus[J]. Front Neurol 12:630681
Cocco M, Garella D, Stilo AD et al (2014) Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis[J]. J Med Chem 57(24):10366–10382
Wu C, Lu W, Zhang Y et al (2019) Inflammasome activation triggers blood clotting and host death through pyroptosis[J]. Immun 50(6):1401-1411.e4
Wellington M, Koselny K, Sutterwala FS et al (2014) Candida albicans triggers NLRP3-mediated pyroptosis in macrophages[J]. Eukaryot Cell 13(2):329–340
Xie J, Bi B, Qin Y, Dong W, Zhong J, Li M, Cheng Y, Xu J, Wang H (2020) Inhibition of phosphodiesterase-4 suppresses HMGB1/RAGE signaling pathway and NLRP3 inflammasome activation in mice exposed to chronic unpredictable mild stress. Brain Behav Immun 92:67–77
Kang R, Hou W, Zhang Q et al (2014) RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer[J]. Cell Death Dis 5(10):e1480
Koivu H, Takakubo Y, Mackiewicz Z et al (2015) Autoinflammation around AES total ankle replacement implants[J]. Foot Ankle Int 36(12):1455–1462
Funding
This study was supported by the Zhejiang Natural Science Foundation (LGF20C9003) and Science and Technology Plan Project of Jiaxing (2021AD30118).
Author information
Authors and Affiliations
Contributions
Chenyang Han and Liping Zhai: design and operation of the experiment. Heping Shen: operation of animal experiment and data processing. Qiaobing Guan: the proposal of the subject, the design of the experimental process, and the whole process guidance.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
The study was approved by Ethics Committee.
Consent for Publication
All authors approved to publish the article.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, C., Zhai, L., Shen, H. et al. Advanced Glycation End-Products (AGEs) Promote Endothelial Cell Pyroptosis Under Cerebral Ischemia and Hypoxia via HIF-1α-RAGE-NLRP3. Mol Neurobiol 60, 2355–2366 (2023). https://doi.org/10.1007/s12035-023-03228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03228-8