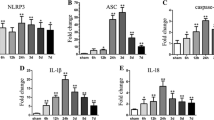
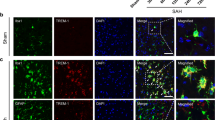
Abstract
Intracerebral hemorrhage (ICH) is characterized by poor prognosis and high mortality rates. To date, satisfactory therapeutic approaches for ICH remain limited, so it is urgently needed to develop a safer and more effective prescription. Secondary inflammatory response has been acknowledged as an aggravating factor to neurological deterioration after ICH. As a component of inflammasome sensors, absent in melanoma 2 (AIM2) plays an important role in the neuroinflammation process. Here, ozanimod, a novel selective sphingosine 1-phosphate receptor modulator, has gained much attention, which alleviates the resultant neuroinflammation and improves functional recovery derived from ICH. In this study, ozanimod improved neurological functions of ICH mice via reduction of hematoma size. Furthermore, both microglial and AIM2 inflammasome activations were reversed by ozanimod, which are confirmed by the downregulation of related inflammatory proteins and cytokines (IL-1β, IL-6, and TNF-α), coupled with the upregulation of SIRT3, by leveraging the Western blot and enzyme-linked immunosorbent assay. Additionally, we find that ozanimod decreases nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) expression. Notably, in vitro cell experiments induced by lipopolysaccharide confirms that the anti-inflammatory effect of ozanimod could be abolished by the SIRT3 inhibitor. In conclusion, these results indicate that ozanimod mitigates ICH-induced secondary inflammatory responses by modulating AIM2 inflammasome mediated by SIRT3/NF-κB/AIM2 pathway. This demonstrates ozanimod orchestrates ICH-induced neuroinflammation and could be a targeted therapy for improving prognosis of ICH.







Similar content being viewed by others
Data Availability
All datasets in this manuscript are available from the corresponding author upon reasonable request.
References
Schrag M, Kirshner H (2020) Management of intracerebral hemorrhage: JACC Focus Seminar. J Am Coll Cardiol 75(15):1819–1831. https://doi.org/10.1016/j.jacc.2019.10.066
Ren H, Han R, Chen X, Liu X, Wan J, Wang L, Yang X, Wang J (2020) Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J Cereb Blood Flow Metab 40(9):1752–1768. https://doi.org/10.1177/0271678X20923551
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, Che F, Chen X, Ren H, Hong M, Wang J (2019) Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol 178:101610. https://doi.org/10.1016/j.pneurobio.2019.03.003
Lan X, Han X, Li Q, Yang QW, Wang J (2017) Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol 13(7):420–433. https://doi.org/10.1038/nrneurol.2017.69
Wang J, Dore S (2007) Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 27(5):894–908. https://doi.org/10.1038/sj.jcbfm.9600403
Xi G, Strahle J, Hua Y, Keep RF (2014) Progress in translational research on intracerebral hemorrhage: is there an end in sight? Prog Neurobiol 115:45–63. https://doi.org/10.1016/j.pneurobio.2013.09.007
Vafaee F, Zangiabadi N, Pour FM, Dehghanian F, Asadi-Shekaari M, Afshar HK (2012) Neuroprotective effects of the immunomodulatory drug Setarud on cerebral ischemia in male rats. Neural Regen Res 7(27):2085–2091. https://doi.org/10.3969/j.issn.1673-5374.2012.27.001
Rahmati M, Keshvari M, Mirnasouri R, Chehelcheraghi F (2021) Exercise and Urtica dioica extract ameliorate hippocampal insulin signaling, oxidative stress, neuroinflammation, and cognitive function in STZ-induced diabetic rats. Biomed Pharmacother 139:111577. https://doi.org/10.1016/j.biopha.2021.111577
Bisht R, Joshi BC, Kalia AN, Prakash A (2017) Antioxidant-rich fraction of urtica dioica mediated rescue of striatal mito-oxidative damage in MPTP-induced behavioral, cellular, and neurochemical alterations in rats. Mol Neurobiol 54(7):5632–5645. https://doi.org/10.1007/s12035-016-0084-z
Cartier A, Hla T (2019) Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366 (6463). https://doi.org/10.1126/science.aar5551
Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A, Blain M, Healy L, Antel J, Quintana FJ (2017) Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A 114(8):2012–2017. https://doi.org/10.1073/pnas.1615413114
Lu L, Barfejani AH, Qin T, Dong Q, Ayata C, Waeber C (2014) Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain Res 1555:89–96. https://doi.org/10.1016/j.brainres.2014.01.048
Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD (2014) Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol 71(9):1092–1101. https://doi.org/10.1001/jamaneurol.2014.1065
Chiba K (2005) FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther 108(3):308–319. https://doi.org/10.1016/j.pharmthera.2005.05.002
Qin C, Fan WH, Liu Q, Shang K, Murugan M, Wu LJ, Wang W, Tian DS (2017) Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48(12):3336–3346. https://doi.org/10.1161/STROKEAHA.117.018505
Gold R, Comi G, Palace J, Siever A, Gottschalk R, Bijarnia M, von Rosenstiel P, Tomic D, Kappos L, Investigators FS (2014) Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol 261(2):267–276. https://doi.org/10.1007/s00415-013-7115-8
Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, Cravets M, Olson A, Frohna PA, Selmaj KW (2016) Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. The Lancet Neurology 15(4):373–381. https://doi.org/10.1016/s1474-4422(16)00018-1
Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, Hartung H-P, Montalban X, Kubala Havrdová E, Cree BAC, Sheffield JK, Minton N, Raghupathi K, Huang V, Kappos L (2019) Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. The Lancet Neurology 18(11):1021–1033. https://doi.org/10.1016/s1474-4422(19)30238-8
Comi G, Kappos L, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, Hartung H-P, Montalban X, Kubala Havrdová E, Cree BAC, Sheffield JK, Minton N, Raghupathi K, Ding N, Cohen JA (2019) Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. The Lancet Neurology 18(11):1009–1020. https://doi.org/10.1016/s1474-4422(19)30239-x
Sandborn WJ, Feagan BG, Wolf DC, D’Haens G, Vermeire S, Hanauer SB, Ghosh S, Smith H, Cravets M, Frohna PA, Aranda R, Gujrathi S, Olson A, Group TS (2016) Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 374(18):1754–1762. https://doi.org/10.1056/NEJMoa1513248
Wang F, Zhang X, Liu Y, Li Z, Wei R, Zhang Y, Zhang R, Khan S, Yong VW, Xue M (2022) Neuroprotection by ozanimod following intracerebral hemorrhage in mice. Front Mol Neurosci 15:927150. https://doi.org/10.3389/fnmol.2022.927150
Yuan B, Zhou XM, You ZQ, Xu WD, Fan JM, Chen SJ, Han YL, Wu Q, Zhang X (2020) Inhibition of AIM2 inflammasome activation alleviates GSDMD-induced pyroptosis in early brain injury after subarachnoid haemorrhage. Cell Death Dis 11(1):76. https://doi.org/10.1038/s41419-020-2248-z
Li Q, Cao Y, Dang C, Han B, Han R, Ma H, Hao J, Wang L (2020) Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. Mol Med 12(4):e11002. https://doi.org/10.15252/emmm.201911002
Franke M, Bieber M, Kraft P, Weber ANR, Stoll G, Schuhmann MK (2021) The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun 92:221–231. https://doi.org/10.1016/j.bbi.2020.12.009
Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM, Brough D (2015) AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A 112(13):4050–4055. https://doi.org/10.1073/pnas.1419090112
Kim H, Seo JS, Lee SY, Ha KT, Choi BT, Shin YI, Ju Yun Y, Shin HK (2020) AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun 87:765–776. https://doi.org/10.1016/j.bbi.2020.03.011
Krafft PR, Rolland WB, Duris K, Lekic T, Campbell A, Tang J, Zhang JH (2012) Modeling intracerebral hemorrhage in mice: injection of autologous blood or bacterial collagenase. J Vis Exp 67:e4289. https://doi.org/10.3791/4289
Chang CF, Goods BA, Askenase MH, Hammond MD, Renfroe SC, Steinschneider AF, Landreneau MJ, Ai Y et al (2018) Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest 128(2):607–624. https://doi.org/10.1172/JCI95612
Caballero-Garrido E, Pena-Philippides JC, Galochkina Z, Erhardt E, Roitbak T (2017) Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system. Behav Brain Res 331:282–296. https://doi.org/10.1016/j.bbr.2017.05.042
Guido C. Koopmans, 3 Ronald Deumens,1 Wiel M.M. Honig,1,3 Frank P.T. Hamers,2,4 Harry W.M. Steinbusch,1 and Elbert a.j. Joosten1,3 (2005) The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. Journal Of Neurotrauma Volume 22 (2)
Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC et al (2017) An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun 8(1):533. https://doi.org/10.1038/s41467-017-00583-8
Ren H, Kong Y, Liu Z, Zang D, Yang X, Wood K, Li M, Liu Q (2018) Selective NLRP3 (pyrin domain-containing protein 3) Inflammasome inhibitor reduces brain injury after intracerebral hemorrhage. Stroke 49(1):184–192. https://doi.org/10.1161/STROKEAHA.117.018904
Zhao L, Chen S, Sherchan P, Ding Y, Zhao W, Guo Z, Yu J, Tang J, Zhang JH (2018) Recombinant CTRP9 administration attenuates neuroinflammation via activating adiponectin receptor 1 after intracerebral hemorrhage in mice. J Neuroinflammation 15(1):215. https://doi.org/10.1186/s12974-018-1256-8
Ju HQ, Ying H, Tian T, Ling J, Fu J, Lu Y, Wu M, Yang L et al (2017) Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat Commun 8:14437. https://doi.org/10.1038/ncomms14437
Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z (2018) MicroRNA-21 overexpression promotes the neuroprotective efficacy of mesenchymal stem cells for treatment of intracerebral hemorrhage. Front Neurol 9:931. https://doi.org/10.3389/fneur.2018.00931
Rahmati M, Rashno A (2021) Automated image segmentation method to analyse skeletal muscle cross section in exercise-induced regenerating myofibers. Sci Rep 11(1):21327. https://doi.org/10.1038/s41598-021-00886-3
Rahmati M, Taherabadi SJ (2021) The effects of exercise training on Kinesin and GAP-43 expression in skeletal muscle fibers of STZ-induced diabetic rats. Sci Rep 11(1):9535. https://doi.org/10.1038/s41598-021-89106-6
Chen Y, Chen S, Chang J, Wei J, Feng M, Wang R (2021) Perihematomal edema after intracerebral hemorrhage: an update on pathogenesis, risk factors, and therapeutic advances. Front Immunol 12:740632. https://doi.org/10.3389/fimmu.2021.740632
Singh SD, Pasi M, Schreuder F, Morotti A, Senff JR, Warren AD, McKaig BN, Schwab K et al (2021) Computed tomography angiography spot sign, hematoma expansion, and functional outcome in spontaneous cerebellar intracerebral hemorrhage. Stroke 52(9):2902–2909. https://doi.org/10.1161/STROKEAHA.120.033297
Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ (2020) Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 22(1 1):29–46. https://doi.org/10.5853/jos.2019.02236
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang S, Deng X, Xie Z, Zheng S (2017) Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation 14(1):119. https://doi.org/10.1186/s12974-017-0895-5
So D, Shin HW, Kim J, Lee M, Myeong J, Chun YS, Park JW (2018) Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene 37(38):5191–5204. https://doi.org/10.1038/s41388-018-0339-4
Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, Billings FTT, Verdin E et al (2020) Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res 126(4):439–452. https://doi.org/10.1161/CIRCRESAHA.119.315767
Proia RL, Hla T (2015) Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest 125(4):1379–1387. https://doi.org/10.1172/JCI76369
Hetze S, Romer C, Teufelhart C, Meisel A, Engel O (2012) Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J Neurosci Methods 206(1):7–14. https://doi.org/10.1016/j.jneumeth.2012.02.001
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219. https://doi.org/10.1002/ana.24070
Yang Z, Zhong L, Xian R, Yuan B (2015) MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol 65(2):267–276. https://doi.org/10.1016/j.molimm.2014.12.018
Yuan B, Shen H, Lin L, Su T, Zhong S, Yang Z (2015) Recombinant adenovirus encoding NLRP3 RNAi attenuate inflammation and brain injury after intracerebral hemorrhage. J Neuroimmunol 287:71–75. https://doi.org/10.1016/j.jneuroim.2015.08.002
Yao ST, Cao F, Chen JL, Chen W, Fan RM, Li G, Zeng YC, Jiao S et al (2017) NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J Mol Neurosci 61(3):385–395. https://doi.org/10.1007/s12031-016-0874-9
Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD (2009) Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab 29(3):534–544. https://doi.org/10.1038/jcbfm.2008.143
de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW (2009) Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 29(7):1251–1261. https://doi.org/10.1038/jcbfm.2009.46
Yao S, Li L, Sun X, Hua J, Zhang K, Hao L, Liu L, Shi D, Zhou H (2019) FTY720 inhibits MPP(+)-induced microglial activation by affecting NLRP3 inflammasome activation. J Neuroimmune Pharmacol 14(3):478–492. https://doi.org/10.1007/s11481-019-09843-4
She DT, Jo DG, Arumugam TV (2017) emerging roles of sirtuins in ischemic stroke. Transl Stroke Res. https://doi.org/10.1007/s12975-017-0544-4
Sidorova-Darmos E, Wither RG, Shulyakova N, Fisher C, Ratnam M, Aarts M, Lilge L, Monnier PP et al (2014) Differential expression of sirtuin family members in the developing, adult, and aged rat brain. Front Aging Neurosci 6:333. https://doi.org/10.3389/fnagi.2014.00333
Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, Waclawiw MA, Han K et al (2015) Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest 125(12):4592–4600. https://doi.org/10.1172/JCI83260
Dong X, He Y, Ye F, Zhao Y, Cheng J, Xiao J, Yu W, Zhao J, Sai Y, Dan G, Chen M, Zou Z (2021) Vitamin D3 ameliorates nitrogen mustard-induced cutaneous inflammation by inactivating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. Clin Transl Med 11(2):e312. https://doi.org/10.1002/ctm2.312
Zhang T, Fang Z, Linghu KG, Liu J, Gan L, Lin L (2020) Small molecule-driven SIRT3-autophagy-mediated NLRP3 inflammasome inhibition ameliorates inflammatory crosstalk between macrophages and adipocytes. Br J Pharmacol 177(20):4645–4665. https://doi.org/10.1111/bph.15215
Acknowledgements
We appreciate the technical support of Dr. Wang ZY from the Institute of Health Science, China Medical University.
Funding
This work was sponsored by the National Natural Science Foundation of China (81971125, 82001234).
Author information
Authors and Affiliations
Contributions
XL and ZH conceived and designed the experiment; JS, HZ, and LW implemented the molecular biology experiment and neurobehavioral tests; XL processed the data and composed the draft manuscript. ZH, JS, and WZ revised the manuscript. All authors discussed the results and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the guidelines for the Care and Use of Laboratory Animals. Approval was granted by the China Medical University Animal Ethics Committee.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhang, H., Zheng, W. et al. Ozanimod-Dependent Activation of SIRT3/NF-κB/AIM2 Pathway Attenuates Secondary Injury After Intracerebral Hemorrhage. Mol Neurobiol 60, 1117–1131 (2023). https://doi.org/10.1007/s12035-022-03137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03137-2