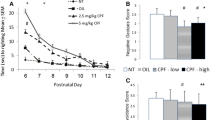
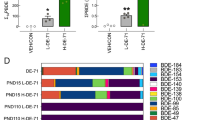
Abstract
Based on previous reports, exposure to pesticides could be linked to the prevalence increase of autism spectrum disorders (ASD). Gestational exposure to chlorpyrifos (CPF) has been associated with ASD diagnosis in humans and ASD-like behaviors in rodents. However, ASD severity degree results from the complex relationship between genetic background and environmental factors. Thus, animals with a genetic vulnerability and prenatally exposed to CPF could have a more severe ASD-like phenotype. Fragile X syndrome is one of the most common monogenic causes of ASD, characterized by a mutation in the X chromosome which alters the expression of the fragile X mental retardation protein (FMRP). Based on this, some fmr1 knockout (KO) rodent models have been developed to study the physiological and genetic basis of ASD. Both fmr1-KO and wild-type male rats (F2 generation) were used in the present study. F1 pregnant females were randomly exposed to 1 mg/kg/mL/day of CPF (s.c.) from GD12.5–15.5 or vehicle. Different behavioral, developmental, and molecular variables were analyzed in F2 males. KO rats were heavier, emitted altered USVs, were socially inefficient, reacted more to a novel stimulus, were hyperactive when exploring a new context, but hypoactive when exploring anxiety-inducing environments, and had an upregulated hippocampal expression of the grin2c gene. When exposed to low doses of CPF during gestation, these KO rats showed decreased climbing capacity, dysfunctional social interaction, and increased hippocampal expression for kcc1 and 5ht2c genes. Gestational CPF exposure increased the ASD-like phenotype in those animals with a genetic vulnerability, although its effect was less generalized than expected. It is the first time that this additive effect of CPF exposure and the fmr1-KO genetic vulnerability model is explored concerning social traits or any other behavior.










Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
Anxiety index
- ANOVA:
-
Analysis of the variance
- APOE:
-
Apolipoprotein E
- ASD:
-
Autism spectrum disorders
- CA:
-
Closed arm
- CPF:
-
Chlorpyrifos
- FSX:
-
X fragile syndrome
- GD:
-
Gestational day
- KO:
-
Knockout
- NOR:
-
Novel object recognition
- OA:
-
Open arm
- OP:
-
Organophosphate
- PM:
-
Plus maze
- PND:
-
Postnatal day
- RT-qPCR:
-
Retro-transcription-quantitative polymerase chain reaction
- S1:
-
Stranger 1
- S2:
-
Stranger 2
- SI:
-
Social index
- SNI:
-
Social novelty index
- USV:
-
Ultrasound vocalization
- WT:
-
Wild-type
- 3-CT:
-
3 Chambers test
References
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Christ SE, Holt DD, White DA, Green L (2007) Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord 37(6):1155–1165. https://doi.org/10.1007/s10803-006-0259-y
Whyatt C, Craig C (2013) Sensory-motor problems in autism. Front Integr Neurosci 7 https://doi.org/10.3389/fnint201300051
Vasa RA, Mazurek MO (2015) An update on anxiety in youth with autism spectrum disorders. Curr Opin Psychiatry 28(2):83–90. https://doi.org/10.1097/YCO0000000000000133
Choi L, An JY (2021) Genetic architecture of autism spectrum disorder: lessons from large-scale genomic studies. Neurosci Biobehav Rev 128:244–257. https://doi.org/10.1016/J.NEUBIOREV.2021.06.028
Chaste P, Leboyer M (2012) Autism risk factors: genes environment and gene-environment interactions. Dialogues Clin Neurosci 2012,14(3) 281. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3513682/
Karimi P, Kamali E, Mousavi SM, Karahmadi M (2017) Environmental factors influencing the risk of autism In. J Res Med Sci 22(1) Isfahan University of Medical Sciences (IUMS) https://doi.org/10.4103/1735–1995200272
Tessari L, Angriman M, Díaz-Román A, Zhang J, Conca A, Cortese S (2020) Association between exposure to pesticides and ADHD or autism spectrum disorder: a systematic review of the literature. J Atten Disord https://doi.org/10.1177/1087054720940402
Biosca-Brull J, Pérez-Fernández C, Mora S, Carrillo B, Pinos H, Conejo NM, Collado P, Arias JL, Martín-Sánchez F, Sánchez-Santed F, Colomina MT (2021) Relationship between autism spectrum disorder and pesticides: a systematic review of human and preclinical models. Int J Environ Res Public Health 18(10):5190. https://doi.org/10.3390/IJERPH18105190
Morales-Navas M, Castaño-Castaño S, Pérez-Fernández C, Sánchez-Gil A, Colomina MT, Leinekugel X, Sánchez-Santed F (2020) Similarities between the effects of prenatal chlorpyrifos and valproic acid on ultrasonic vocalization in infant Wistar rats. Int J Environ Res Public Health 17(17):6376. https://doi.org/10.3390/IJERPH17176376
Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS (2008) Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 38(sup2):1–125. https://doi.org/10.1080/10408440802272158
Lan A, Stein D, Portillo M, Toiber D, Kofman O (2019) Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav Brain Funct 15(1):2. https://doi.org/101186/s12993-019-0153-3
Lan A, Kalimian M, Amram B, Kofman O (2017) Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environ Health 16(1):43. https://doi.org/10.1186/s12940-017-0251-3
Abreu AC, Navas MM, Fernández CP, Sánchez-Santed F, Fernández I (2021) NMR-based metabolomics approach to explore brain metabolic changes induced by prenatal exposure to autism-inducing chemicals. ACS Chem Biol 16(4):753–765. https://doi.org/10.1021/ACSCHEMBIO.1C00053
De Felice A, Scattoni ML, Ricceri L, Calamandrei G (2015) Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One 10(3):e0121663. https://doi.org/10.1371/journal.pone.0121663
De Felice A, Anita G, Calamandrei G, Minghetti L (2016) Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 13(1):149. https://doi.org/10.1186/s12974-016-0617-4
Basaure P, Guardia-Escote L, Biosca-Brull J, Blanco J, Cabré M, Peris-Sampedro F, Sánchez-Santed F, Domingo JL, Colomina MT (2019) Exposure to chlorpyrifos at different ages triggers APOE genotype-specific responses in social behavior body weight and hypothalamic gene expression. Environ Res 178:108684. https://doi.org/10.1016/J.ENVRES.2019.108684
Mullen BR, Khialeeva E, Hoffman DB, Ghiani CA, Carpenter EM (2013) Decreased reelin expression and organophosphate pesticide exposure alters mouse behaviour and brain morphology. ASN Neuro 5(1):AN20120060. https://doi.org/10.1042/AN20120060
Fyke W, Velinov M (2021) FMR1 and autism an intriguing connection revisited. Genes 12(8). https://doi.org/10.3390/GENES12081218
Ellenbroek B, Youn J (2016) Rodent models in neuroscience research: Is it a rat race? DMM Dis Models Mech 9(10):1079–1087. https://doi.org/10.1242/dmm.026120
Siegel-Ramsay JE, LRomaniuk L, Whalley HC, Roberts N, Holly Branigan H, Stanfield AC, Lawrie SM, Dauvermann MR (2021) Glutamate and functional connectivity - support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res Neuroimaging 313 https://doi.org/10.1016/JPSCYCHRESNS2021111302
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Garrido-Cárdenas JA, Colomina MT, Giménez E, Sánchez-Santed F (2020) Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: a locomotor pharmacological brain gene expression and gut microbiome analysis. Food Chem Toxicol 135 https://doi.org/10.1016/jfct2019110865
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Colomina MT, Giménez E, Sánchez-Santed F (2020) Postnatal exposure to low doses of chlorpyrifos induces long-term effects on 5C-SRTT learning and performance cholinergic and GABAergic systems and BDNF expression. Exp Neurol 202:113356. https://doi.org/10.1016/jexpneurol2020113356
Venerosi A, Ricceri L, Scattoni ML, Calamandrei G (2009) Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ Health Glob Access Sci Source 8(1):12. https://doi.org/10.1186/1476-069X-8-12
Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Colomina MT, Giménez E, Sánchez-Santed F (2021) Pesticides and aging: preweaning exposure to chlorpyrifos induces a general hypomotricity state in late-adult rats. NeuroToxicol 86:69–77. https://doi.org/10.1016/JNEURO202107002
Liu ZH, Chuang DM, Smith CB (2011) Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol 14(5):618–630. https://doi.org/10.1017/S1461145710000520
Leboucher A, Bermudez-Martin P, Mouska X, Amri E-Z, Pisani DF, Davidovic L (2019) Fmr1-deficiency impacts body composition skeleton and bone microstructure in a mouse model of fragile X syndrome. Front Endocrinol 10:678. https://doi.org/10.3389/fendo201900678
Qin M, Xia Z, Huang T, Smith CB (2011) Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in fmr1 knockout mice. Neurosci 194:282. https://doi.org/10.1016/JNEUROSCIENCE201106047
Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF (2007) Correction of fragile X syndrome in mice. Neuron 56(6):955. https://doi.org/10.1016/J.NEURON.2007.12.001
de Vries BBA, Fryns JP, Butler MG, Canziani F, Wesby-van Swaay E, van Hemel JO, Oostra BA, Halley DJJ, Niermeijer MF (1993) Clinical and molecular studies in fragile X patients with a Prader-Willi-like phenotype. J Med Genet 30(9):761–766. https://doi.org/10.1136/jmg.30.9.761
Schrander-Stumpel C, Gerver W‐J, Engelen J, Mulder H, Fryns J‐P (1994) Prader-Willi-like phenotype in fragile X syndrome. Clin Genet 45(4):175–180. https://doi.org/10.1111/j1399-00041994tb04018x
McLennan Y, Polussa J, Tassone F, Hagerman R (2011) Fragile X Syndrome. Curr Genomics 12(3):216–224. https://doi.org/10.2174/138920211795677886
Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA, Crnic LS, Strupp BJ (2006) Attentional dysfunction impulsivity and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci 120(6):1367–1379. https://doi.org/10.1037/0735-704412061367
Bausch AE, Ehinger R, Straubinger J, Zerfass P, Nann Y, Lukowski R (2018) Loss of sodium-activated potassium channel slack and FMRP differentially affect social behavior in mice. Neuroscience 384:361–374. https://doi.org/10.1016/j.neuroscience.2018.05.040
Roy S, Zhao Y, Allensworth M, Farook MF, LeDoux MS, Reiter LT, Heck DH (2011) Comprehensive motor testing in fmr1-KO mice exposes temporal defects in oromotor coordination. Behav Neurosci 125(6):962–969. https://doi.org/10.1037/a0025920
Schiavi S, Carbone E, Melancia F, Buzzelli V, Manduca A, Campolongo P, Pallottini V, Trezza V (2020) Perinatal supplementation with omega-3 fatty acids corrects the aberrant social and cognitive traits observed in a genetic model of autism based on FMR1 deletion in rats. Nutr Neurosci https://doi.org/10.1080/1028415X20201819107
Tian Y, Yang C, Shang S, Cai Y, Deng X, Zhang J, Shao F, Zhu D, Liu Y, Chen G, Liang J, Sun Q, Qiu Z, Zhang C (2017) Loss of FMRP impaired hippocampal long-term plasticity and spatial learning in rats. Front Mol Neurosci 10 https://doi.org/10.3389/fnmol201700269
Asiminas A, Jackson AD, Louros SR, Till SM, Spano T, Dando O, Bear MF, Chattarji S, Hardingham GE, Osterweil EK, Wyllie DJA, Wood ER, Kind PC (2019) Sustained correction of associative learning deficits after brief early treatment in a rat model of fragile X syndrome. Sci Transl Med 11(494). https://doi.org/10.1126/scitranslmed.aao0498
Saxena K, Webster J, Hallas-Potts A, Mackenzie R, Spooner PA, Thomson D, Kind P, Chatterji S, Morris RGM (2018) Experiential contributions to social dominance in a rat model of fragile-X syndrome. Proc R Soc B Biol Sci 285:1880. https://doi.org/10.1098/rspb20180294
Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R (2012) Group i metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology 219(1):47–58. https://doi.org/10.1007/s00213-011-2375-4
Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV (2010) Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav 9(6):562–574. https://doi.org/10.1111/J.1601-183X.2010.00585.X
Renoux AJ, Sala-Hamrick KJ, Carducci NM, Frazer M, Halsey KE, Sutton MA, Dolan DF, Murphy GG, Todd PK (2014) Impaired sensorimotor gating in Fmr1 knock out and fragile X premutation model mice. Behav Brain Res 267:42–45. https://doi.org/10.1016/jbbr201403013
de Vrij FMS, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R (2008) Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis 31(1):127–132. https://doi.org/10.1016/j.nbd.2008.04.002
Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R (2006) Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between fragile X-related proteins. Human Mol Genet 15(12):1984–1994. https://doi.org/10.1093/hmg/ddl121
Kokash J, Alderson EM, Reinhard SM, Crawford CA, Binder DK, Ethell IM, Razak KA (2019) Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res 1719:24–29. https://doi.org/10.1016/jbrainres201905029
Perez-Fernandez C, Morales-Navas M, Aguilera-Sáez LM, Abreu AC, Guardia-Escote L, Fernández I, Garrido-Cárdenas JA, Colomina MT, Giménez E, Sánchez-Santed F (2020) Medium and long-term effects of low doses of chlorpyrifos during the postnatal preweaning developmental stage on sociability dominance gut microbiota and plasma metabolites. Environ Res 184:109341. https://doi.org/10.1016/jenvres2020109341
Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA (2012) Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of fragile X Syndrome. Brain Res 1439:7–14. https://doi.org/10.1016/jbrainres201112041
Toledo MA, Wen TH, Binder DK, Ethell IM, Razak KA (2019) Reversal of ultrasonic vocalization deficits in a mouse model of fragile X syndrome with minocycline treatment or genetic reduction of MMP-9. Behav Brain Res 372:112068. https://doi.org/10.1016/jbbr2019112068
Lai JKY, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA (2014) Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res 259:119–130. https://doi.org/10.1016/jbbr201310049
Belagodu AP, Johnson AM, Galvez R (2016) Characterization of ultrasonic vocalizations of fragile X mice. Behav Brain Res 310:76–83. https://doi.org/10.1016/j.bbr.2016.04.016
Nolan SO, Hodges SL, Lugo JN (2020) High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes Brain Behav 19(2) https://doi.org/10.1111/gbb12611
Hodges SL, Nolan SO, Reynolds CD, Lugo JN (2017) Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice. Behav Brain Res 332:50–58. https://doi.org/10.1016/jbbr201705052
Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE (2011) Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One 6(2) https://doi.org/10.1371/journalpone0017073
Saldarriaga W, Lein P, Teshima LYG, Isaza C, Rosa L, Polyak A, Hagerman R, Girirajan S, Silva M, Tassone F (2016) Phenobarbital use and neurological problems in FMR1 premutation carriers. Neurotoxicology 53:141. https://doi.org/10.1016/JNEURO201601008
Costa L, Sardone LM, Bonaccorso CM, D’Antoni S, Spatuzza M, Gulisano W, Tropea MR, Puzzo D, Leopoldo M, Lacivita E, Catania MV, Ciranna L (2018) Activation of serotonin 5-HT7 receptors modulates hippocampal synaptic plasticity by stimulation of adenylate cyclases and rescues learning and behavior in a mouse model of fragile X syndrome. Front Mol Neurosci 0:353. https://doi.org/10.3389/FNMOL.2018.00353
Prieto M, Folci A, Poupon G, Schiavi S, Buzzelli V, Pronot M, François U, Pousinha P, Lattuada N, Abelanet S, Castagnola S, Chafai M, Khayachi A, Gwizdek C, Brau F, Deval E, Francolini M, Bardoni B, Humeau Y, Martin S (2021) Missense mutation of Fmr1 results in impaired AMPAR-mediated plasticity and socio-cognitive deficits in mice. Nat Commun 12(1):1–15. https://doi.org/10.1038/s41467-021-21820-1
Lieb-Lundell C (2016) Three faces of fragile X. Physical Therapy 96(11):1782–1790. https://doi.org/10.2522/PTJ20140430
Garrido D, Petrova D, Watson LR, Garcia-Retamero R, Carballo G (2017) Language and motor skills in siblings of children with autism spectrum disorder: a meta-analytic review. Autism Res 10(11):1737–1750. https://doi.org/10.1002/AUR.1829
Hayashi ML, Rao BSS, Seo J-S, Choi H-S, Dolan BM, Choi S-Y, Chattarji S, Tonegawa S (2007) Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A 104(27):11489. https://doi.org/10.1073/PNAS0705003104
Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, Åkerman K, Castrén E, Castrén ML (2012) Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes Brain Behav 11(5):513–523. https://doi.org/10.1111/J1601-183X201200784X
Uutela M, Lindholm J, Rantamäki T, Umemori J, Hunter K, Võikar V, Castrén ML (2014) Distinctive behavioral and cellular responses to fluoxetine in the mouse model for fragile X syndrome. Front Cell Neurosci 8 https://doi.org/10.3389/FNCEL201400150
Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE, Razak KA, Ethell DW, Ethell IM (2013) Long-lasting effects of minocycline on behavior in young but not adult fragile X mice. Neuroscience 246:186–198. https://doi.org/10.1016/J.NEUROSCIENCE.2013.04.058
Oddi D, Subashi E, Middei S, Bellocchio L, Lemaire-Mayo V, Guzmán M, Crusio WE, D’Amato FR, Pietropaolo S (2015) Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology 40(5):1113. https://doi.org/10.1038/NPP2014291
Qiu G, Chen S, Guo J, Wu J, Yi YH (2016) Alpha-asarone improves striatal cholinergic function and locomotor hyperactivity in Fmr1 knockout mice. Behav Brain Res 312:212–218. https://doi.org/10.1016/JBBR201606024
Schaefer TL, Davenport MH, Grainger LM, Robinson CK, Earnheart AT, Stegman MS, Lang AL, Ashworth AA, Molinaro G, Huber KM, Erickson CA (2017) Acamprosate in a mouse model of fragile X syndrome: modulation of spontaneous cortical activity ERK1/2 activation locomotor behavior and anxiety. J Neurodevelopmental Disord 9(1) https://doi.org/10.1186/S11689-017-9184-Y
Chatterjee M, Kurup PK, Lundbye CJ, Hugger Toft AK, Kwon J, Benedict J, Kamceva M, Banke TG, Lombroso PJ (2018) STEP inhibition reverses behavioral electrophysiologic and synaptic abnormalities in Fmr1 KO mice. Neuropharmacology 128:43–53. https://doi.org/10.1016/J.NEUROPHARM.2017.09.026
Pirbhoy PS, Rais M, Lovelace JW, Woodard W, Razak KA, Binder DK, Ethell IM (2020) Acute pharmacological inhibition of matrix metalloproteinase-9 activity during development restores perineuronal net formation and normalizes auditory processing in Fmr1 KO mice. J Neurochem 155(5):538–558. https://doi.org/10.1111/JNC15037
Kozono NO, Kamura A, Honda S, Matsumoto M, Mihara T (2020) Gamma power abnormalities in a Fmr1-targeted transgenic rat model of fragile X syndrome. Sci Rep 10(1) https://doi.org/10.1038/S41598-020-75893-X
Wong H, Hooper AWM, Niibori Y, Lee SJ, Hategan LA, Zhang LZ, Karumuthil-Melethil S, Sally M, Till SM, Peter C, Kind PC, Olivier Danos O, Bruder JT, Hampson DR (2020) Sexually dimorphic patterns in electroencephalography power spectrum and autism-related behaviors in a rat model of fragile X syndrome. Neurobiol Dis 146 https://doi.org/10.1016/JNBD2020105118
Fish EW, Krouse MC, Stringfield SJ, Diberto JF, Robinson JE, Malanga CJ (2013) Changes in sensitivity of reward and motor behavior to dopaminergic glutamatergic and cholinergic drugs in a mouse model of fragile X syndrome. PloS One 8(10). https://doi.org/10.1371/JOURNAL.PONE.0077896
Yu Y, Lin Y, Takasaki Y, Wang C, Kimura H, Xing J, Ishizuka K, Toyama M, Kushima I, Mori D, Arioka Y, Uno Y, Shiino T, Nakamura Y, Okada T, Morikawa M, Ikeda M, Iwata N, Okahisa Y, Ozaki N (2018) Rare loss of function mutations in N-methyl-d-aspartate glutamate receptors and their contributions to schizophrenia susceptibility Translational. Psychiatry 8(1):12. https://doi.org/10.1038/S41398-017-0061-Y
Dölen G, Bear MF (2008) Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586(6):1503–1508. https://doi.org/10.1113/JPHYSIOL.2008.150722
Yau S-Y, Bettio L, Chiu J, Chiu C, Christie BR (2019) Fragile-X syndrome is associated with NMDA receptor hypofunction and reduced dendritic complexity in mature dentate granule cells. Front Mol Neurosci 11 https://doi.org/10.3389/FNMOL201800495
Banke TG, Barria A (2020) Transient enhanced GluA2 expression in young hippocampal neurons of a fragile X mouse model. Front Synaptic Neurosci 12. https://doi.org/10.3389/FNSYN.2020.588295
Bostrom CA, Majaess N-M, Morch K, White E, Eadie BD, Christie BR (2015) Rescue of NMDAR-dependent synaptic plasticity in Fmr1 knock-out mice. Cereb Cortex 25(1):271–279. https://doi.org/10.1093/CERCOR/BHT237
Vieira M, Yong XLH, Roche KW, Anggono V (2020) Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J Neurochem 154(2):121–143. https://doi.org/10.1111/JNC14970
Wold EA, Wild CT, Cunningham KA, Zhou J (2019) Targeting the 5-HT2C receptor in biological context and the current state of 5-HT2C receptor ligand development. Curr Topics Med Chem 19(16):1381. https://doi.org/10.2174/1568026619666190709101449
Prophitt J, Armstrong J, Chen Y, Canal C (2019) Assessment of brain serotonin receptors in an Fmr1 knockout mouse model of fragile X syndrome. FASEB J 33(S1):6671–6671. https://doi.org/10.1096/FASEBJ2019331_SUPPLEMENT6671
Saraf T, Chen Y, Armstrong JL, Prophitt J, Canal C (2021) Evaluation of Serotonin 5-HT1A 5-HT2A and 5-HT2C receptors and the serotonin transporter in an Fmr1 knockout mouse model of fragile X syndrome. FASEB J 35(S1) https://doi.org/10.1096/FASEBJ202135S103781
Garneau AP, Slimani S, Tremblay LE, Fiola MJ, Marcoux AA, Isenring P (2019) K+-Cl- cotransporter 1 (KCC1): A housekeeping membrane protein that plays key supplemental roles in hematopoietic and cancer cells. J Hematol Oncol 12(1). https://doi.org/10.1186/S13045-019-0766-X
Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K (2002) Developmental changes in KCC1 KCC2 and NKCC1 mRNA expressions in the rat brain. Dev Brain Res 139(1):59–66. https://doi.org/10.1016/S0165-3806(02)00536-9
Lydiard RB (2003) The role of GABA in anxiety disorders. J Clin Psychiatry 2003 64(suppl 3).
Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJC, Kendler G, KS, Chen X, (2006) Association between glutamic acid decarboxylase genes and anxiety disorders major depression and neuroticism. Mol Psychiatry 11(8):752–762. https://doi.org/10.1038/sjmp4001845
Engin E, Liu J, Rudolph U (2012) α2-containing GABAA receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol Ther 136(2):142. https://doi.org/10.1016/J.PHARMTHERA.2012.08.006
Myslivecek J, Farar V, Valuskova P (2017) M(4) muscarinic receptors and locomotor activity regulation. Physiol Res 66(Suppl 4):S443–S455. https://doi.org/10.33549/PHYSIOLRES933796
Koshimizu H, Leiter LM, Miyakawa T (2012) M 4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol Brain 5(1):1–10. https://doi.org/10.1186/1756-6606-5-10
Unal G, Bekci H, Cumaoglu A, Yerer MB, Aricioglu F (2020) Alpha 7 nicotinic receptor agonist and positive allosteric modulators improved social and molecular deficits of MK-801 model of schizophrenia in rats. Pharmacol Biochem Behav 193:172916. https://doi.org/10.1016/JPBB2020172916
Halberstadt AL, Powell SB, Geyer MA (2013) Role of the 5-HT2A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology 70:218. https://doi.org/10.1016/JNEUROPHARM201301014
Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB (2009) 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34(8):1958–1967. https://doi.org/10.1038/npp200929
Platzer K, Yuan H, Schütz H, Winschel A, Chen W, Hu C, Kusumoto H, Heyne HO, Helbig KL, Tang S, Willing MC, Tinkle BT, Adams DJ, Depienne C, Keren B, Mignot C, Frengen E, Strømme P, Biskup S, Lemke JR (2017) GRIN2B encephalopathy: novel findings on phenotype variant clustering functional consequences and treatment aspects. J Med Genet 54(7):460–470. https://doi.org/10.1136/JMEDGENET-2016-104509
Narita K, Murata T, Matsuoka S (2016) The ventromedial hypothalamus oxytocin induces locomotor behavior regulated by estrogen. Physiol Behav 164:107–112. https://doi.org/10.1016/JPHYSBEH201605047
Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A (2017) Hippocampal oxytocin receptors are necessary for discrimination of social stimuli Nature. Communications 8(1):1–14. https://doi.org/10.1038/s41467-017-02173-0
Chamoun M, Groleau M, Bhat M (2016) Dose-dependent effect of donepezil administration on long-term enhancement of visually evoked potentials and cholinergic receptor overexpression in rat visual cortex. J Phys Paris 110(1–2):65–74. https://doi.org/10.1016/J.JPHYSPARIS.2016.11.010
Lips KS, Lührmann A, Tschernig T, Stoeger T, Alessandrini F, Grau V, Haberberger RV, Koepsell H, Pabst R, Kummer W (2007) Down-regulation of the non-neuronal acetylcholine synthesis and release machinery in acute allergic airway inflammation of rat and mouse. Life Sci 80(24–25):2263–2269. https://doi.org/10.1016/jlfs200701026
Jameson RR, Seidler FJ, Slotkin TA (2007) Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon, and diazinon. Environ Health Perspect 115(1):65–70. https://doi.org/10.1289/ehp9487
Fujimura J, Nagano M, Suzuki H (2005) Differential expression of GABAA receptor subunits in the distinct nuclei of the rat amygdala. Mol Brain Res 138(1):17–23. https://doi.org/10.1016/j.molbrainres.2005.03.013
Jaenisch N, Witte OW, Frahm C (2010) Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41(3):e151-9. https://doi.org/10.1161/STROKEAHA109570424
Cho H-M, Lee H-A, Kim HY, Lee D-Y, Kim IK (2013) Recruitment of specificity protein 1 by CpG hypomethylation upregulates Na+-K+-2Cl− cotransporter 1 in hypertensive rats. J Hypertens 31(7):1406–1413. https://doi.org/10.1097/HJH.0b013e3283610fed
Kindlundh-Högberg AMS, Svenningsson P, Schiöth HB (2006) Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology 51(4):838–847. https://doi.org/10.1016/jneuropharm200605026
Lau JC, Kroes RA, Moskal JR, Linsenmeier RA (2013) Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol vision 19:1538–1553
Pershina EV, Mikheeva IB, Kamaltdinova ER, Arkhipov VI (2018) Expression of mGlu receptor genes in the hippocampus after intoxication with trimethyltin. J Mol Neurosci 67(2):258–264. https://doi.org/10.1007/S12031-018-1233-9
Hamilton SM, Green JR, Veeraragavan S et al (2014) Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci 128(2):103–109. https://doi.org/10.1037/A0035988
Acknowledgements
We are grateful to Ainhoa Sánchez Gil for its initial help with the F0 mating/breeding protocol
Funding
This study was supported by the Grant PID2020-113812RB-C32, MCIN/AEI/ https://doi.org/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
P-F C made the original conceptualization of the experimental design, completed the animals’ maintenance, behavioral tasks, initial statistical analyses, figures design, and the first version of the manuscript. MM M made most of the gene expression analyzed. M–N M helped with the original conceptualization of the study design, behavioral experiments, and sacrifices. G-E L, C M, and G E completed the genotyping protocol (including optimization of the PCR settings) and helped with the gene expression analyses. CM T and S–S F had the original conceptualization of this set of experiments, completed continuous supervision of the work, get the economical and material resources, and review this manuscript until its last version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The present study is part of the project ES040130002260 and was conducted following the Spanish Royal Decree 53/2013, the European Community Directive (2010/63/EU) for animal research and complies with the ARRIVE guidelines for animal research. The Animal Research Committee of the University of Almeria approved the experiments described here.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perez-Fernandez, C., Matamala Montoya, M., Morales-Navas, M. et al. Influence of Gestational Chlorpyrifos Exposure on ASD-like Behaviors in an fmr1-KO Rat Model. Mol Neurobiol 59, 5835–5855 (2022). https://doi.org/10.1007/s12035-022-02933-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02933-0