Abstract
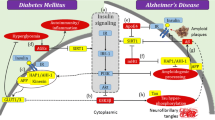
Alzheimer’s disease (AD) is one of the causes of dementia that results from several infections/biological conditions leading to either cell disruption or loss of neuronal communication. Studies have documented the accumulation of two proteins, beta-amyloid (Aβ), which accumulates on the exteriors of neurons, and tau (Tau), which assembles at the interiors of brain cells and is chiefly liable for the progression of the disease. Several molecular and cellular pathways account for the accumulation of amyloid-β and the formation of neurofibrillary tangles, which are phosphorylated variants of Tau protein. Moreover, research has revealed a potential connection between AD and diabetes. It has also been demonstrated that both hypoglycemia and hyperglycemia have a significant role in the development of AD. In addition, SUMO (small ubiquitin-like modifier protein) plays a crucial role in the pathogenesis of AD. SUMOylation is the process by which modification of amyloid precursor protein (APP) and Tau takes place. Furthermore, Drosophila melanogaster has proven to be an efficient model organism in studies to establish the relationship between AD and variations in blood glucose levels. In addition, the review successfully identifies the common pathway that links the effects of fluctuations in glucose levels on AD pathogenesis and advancements.






Similar content being viewed by others
Data availability
Not applicable.
References
Farran CJ, Fogg LG, McCann JJ, Etkin C, Dong X, Barnes LL (2011) Assessing family caregiver skills in managing behavioral symptoms of Alzheimer’s disease. Aging Ment Health 15(4):510–521. https://doi.org/10.1080/13607863.2010.536140
Tumminia A, Vinciguerra F, Parisi M, Frittitta L (2018) Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci 19(11):3306. https://doi.org/10.3390/ijms19113306
Musselman LP, Fink JL, Baranski TJ (2019) Similar effects of high-fructose and high-glucose feeding in a Drosophila model of obesity and diabetes. PLoS One 14(5):e0217096. https://doi.org/10.1371/journal.pone.0217096
Tue NT, Dat TQ, Ly LL, Anh VD, Yoshida H (2020) Insights from Drosophila melanogaster model of Alzheimer’s disease. Front Biosci (Landmark Ed) 25(1):134–146. https://doi.org/10.2741/4798
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008. https://doi.org/10.3389/fphar.2019.01008
Tan CC, Yu JT, Tan L (2014) Biomarkers for preclinical Alzheimer’s disease. J Alzheimers Dis 42(4):1051–1069. https://doi.org/10.3233/JAD-140843
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397(10284):1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Reiss AB, Arain HA, Stecker MM, Siegart NM, Kasselman LJ (2018) Amyloid toxicity in Alzheimer’s disease. Rev Neurosci 29(6):613–627. https://doi.org/10.1515/revneuro-2017-0063
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine 14:5541–5554. Published 2019 Jul 19. https://doi.org/10.2147/IJN.S200490
Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6(11):1054–1061. https://doi.org/10.1038/ncb1104-1054
Kimberly WT, Zheng JB, Guénette SY, Selkoe DJ (2001) The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem 276(43):40288–40292. https://doi.org/10.1074/jbc.C100447200
Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME et al (2008) Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 65(8). https://doi.org/10.1001/archneur.65.8.1102
Goedert M (2015) Alzheimer’s and Parkinson’s diseases: the prion concept with assembled A, tau, and -synuclein. Science 349(6248):1255555–1255555. https://doi.org/10.1126/science.1255555
Tu S, Okamoto S, Lipton SA, Xu H (2014) Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener 14(9):48. https://doi.org/10.1186/1750-1326-9-48
Chen JX, Yan SS (2010) Role of mitochondrial amyloid-beta in Alzheimer’s disease. J Alzheimers Dis 20(2):S569–S578. https://doi.org/10.3233/JAD-2010-100357
Sherrington R, Rogaev EI, Liang Y et al (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 375(6534):754–760
Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of gamma-secretase activity. Nat Cell Biol 5(5):486–488. https://doi.org/10.1038/ncb960
Shen J, Kelleher RJ 3rd. (2007) The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A 104(2)):403–409. https://doi.org/10.1073/pnas.0608332104
Reitz C (2015) Genetic diagnosis and prognosis of Alzheimer’s disease: challenges and opportunities. Expert Rev Mol Diagn 15(3):339–348. https://doi.org/10.1586/14737159.2015.1002469
Cruts M, Theuns J, Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat 33(9):1340–1344
Guerreiro RJ, Gustafson DR, Hardy J (2012) The genetic architecture of Alzheimer’s disease: beyond APP, PSENs and APOE. Neurobiol Aging 33(3):437–456
Misrani A, Tabassum S, Yang L (2021) Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front Aging Neurosci 18(13):617588. https://doi.org/10.3389/fnagi.2021.617588
Swerdlow RH (2018) Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis 62(3):1403–1416. https://doi.org/10.3233/JAD-170585
Moreira PI (2018) Sweet mitochondria: a shortcut to Alzheimer’s disease. J Alzheimers Dis 62:1391–1401
Carvalho C, Cardoso S (2020) Diabetes-Alzheimer’s disease link: targeting mitochondrial dysfunction and redox imbalance. Antioxid Redox Signal. https://doi.org/10.1089/ars.2020.8056
Gallart-Palau X, Lee BST, Adav SS et al (2016) Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer’s disease with cerebrovascular disease. Mol Brain 9:27. https://doi.org/10.1186/s13041-016-0205-7
Luo HB, Xia YY, Shu XJ, Liu ZC, Feng Y, Liu XH, Yu G, Yin G, Xiong YS, Zeng K, Jiang J, Ye K, Wang XC, Wang JZ (2014) SUMOylation at K340 inhibits tau degradation by deregulating its phosphorylation and ubiquitination. Proc Natl Acad Sci U S A 18:16586–16591
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science. 314(5800):777–781. https://doi.org/10.1126/science.1132814
Amos LA (2014) Why do brains need tau (MAPT)? FEBS J 281(21):iv–v. https://doi.org/10.1111/febs.13094
Bloom GS (2014a) Amyloid-β and tau: the trigger and bullet in Alzheimer’s disease pathogenesis. JAMA Neurol 71(4):505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K (1994) Role of abnormallyphosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A 91(12):5562–5566. https://doi.org/10.1073/pnas.91.12.5562
Iqbal K, Grundke-Iqbal I (1991) Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer’s disease. Mol Neurobiol 5(2–4):399–410. https://doi.org/10.1007/BF02935561
Dorval V, Fraser PE (2006) Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem 281(15):9919–9924. https://doi.org/10.1074/jbc.M510127200
Dorval V, Fraser PE (2007) SUMO on the road to neurodegeneration. Biochim Biophys Acta 1773(6):694–706. https://doi.org/10.1016/j.bbamcr.2007.03.017
Takahashi K et al (2008) SUMO-1 immunoreactivity colocalizes with phospho-Tau in APP transgenic mice but not in mutant Tau transgenic mice. Neurosci Lett 441:90–93
Claeysen S, Cochet M, Donneger R, Dumuis A, Bockaert J, Giannoni P (2012) Alzheimer culprits: cellular crossroads and interplay. Cell Signal 24(9):1831–1840. https://doi.org/10.1016/j.cellsig.2012.05.008
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204. https://doi.org/10.1146/annual-neuro-061010-113613
Noble W, Hanger DP, Miller CC, Lovestone S (2013) The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 1(4):83. https://doi.org/10.3389/fneur.2013.00083
Johnson GV, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117(Pt 24):5721–5729. https://doi.org/10.1242/jcs.01558
Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F (2013) Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev 12(1):289–309. https://doi.org/10.1016/j.arr.2012.06.003
Panda C, Voelz C, Habib P, Mevissen C, Pufe T, Beyer C, Gupta S, Slowik A (2021) Aggregated Tau-PHF6 (VQIVYK) potentiates NLRP3 inflammasome expression and autophagy in human microglial cells. Cells. 10(7):1652. https://doi.org/10.3390/cells10071652
Dorval V, Mazzella MJ, Mathews PM, Hay RT, Fraser PE (2007) Modulation of Abeta generation by small ubiquitin-like modifiers does not require conjugation to target proteins. Biochem J 404(2):309–316. https://doi.org/10.1042/BJ20061451
Shalizi A, Gaudillière B, Yuan Z, Stegmüller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A (2006) A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 311(5763):1012–1017. https://doi.org/10.1126/science.1122513
Kota V, Sommer G, Durette C, Thibault P, van Niekerk EA, Twiss JL, Heise T (2016) SUMO-modification of the La protein facilitates binding to mRNA in vitro and in cells. PLoS One 11(5):e0156365. https://doi.org/10.1371/journal.pone.0156365
Scheschonka A, Tang Z, Betz H (2007) Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci 30(3):85–91. https://doi.org/10.1016/j.tins.2007.01.003
Wilkinson KA, Nakamura Y, Henley JM (2010) Targets and consequences of protein SUMOylation in neurons. Brain Res Rev 64(1):195–212. https://doi.org/10.1016/j.brainresrev.2010.04.002
Bohren KM et al (2007) Affinity chromatography of native SUMO proteins using His-tagged recombinant UBC9 bound to Co2+-charged talon resin. Protein Expr Purif 54:289–294
Desterro JM et al (1999) Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. J Biol Chem 274:10618–10624
Okuma T et al (1999) In vitro SUMO-1 modification requires two enzymatic steps, E1 and E2. BiochimBiophys Res Commun 254:693–698
Johnson ES, Blobel G (1997) Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem 272:26799–26802
Rodriguez MS et al (2001) SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 276:12654–12659
Sampson DA et al (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276:21664–21669
Geiss-Friedlander R, Melchior F (2007) Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8:947–956
Mukhopadhyay D, Dasso M (2007) Modification in reverse: the SUMO proteases. Trends Biochem Sci 32:286–295
Zhao XY, Wang DD, Shan Y, Zhu CQ (2013) Potential involvement of abnormal increased SUMO-1 in modulation of the formation of Alzheimer’s disease senile plaques and neuritic dystrophy in APP/PS1 transgenic mice. Sheng Li Xue Bao 65(3):253–262 Chinese
McMillan LE, Brown JT, Henley JM, Cimarosti H (2011) Profiles of SUMO and ubiquitin conjugation in an Alzheimer’s disease model. Neurosci Lett 502(3):201–208. https://doi.org/10.1016/j.neulet.2011.07.045
Yun SM, Cho SJ, Song JC, Song SY, Jo SA, Jo C, Yoon K, Tanzi RE, Choi EJ, Koh YH (2013) SUMO1 modulates Aβ generation via BACE1 accumulation. Neurobiol Aging 34:650–662
Cho SJ, Yun SM, Jo C, Lee DH, Choi KJ, Song JC, Park SI, Kim YJ, Koh YH (2015) SUMO1 promotes Aβ production via the modulation of autophagy. Autophagy. 11(1):100–112. https://doi.org/10.4161/15548627.2014.984283
Ballatore C et al (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672
Dorval V, Fraser PE (2006a) Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem 281:9919–9924
Chen X, Zhang Y, Wang Q, Qin Y, Yang X, Xing Z, Shen Y, Wu H, Qi Y (2021) The function of SUMOylation and its crucial roles in the development of neurological diseases. FASEB J 35(4):e21510. https://doi.org/10.1096/fj.202002702R
Unnikrishnan R, Anjana R, Mohan V (2016) Diabetes mellitus and its complications in India. Nat Rev Endocrinol 12:357–370. https://doi.org/10.1038/nrendo.2016.53
Pradeepa R, Mohan V (2017 Jul) Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr 71(7):816–824. https://doi.org/10.1038/ejcn.2017.40
Susan van D, Beulens JW, Yvonne T. van der S, Grobbee DE, Nealb B (2010) The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 17(1_suppl):s3–s8. https://doi.org/10.1097/01.hjr.0000368191.86614.5a
Tandon RK, Srivastava LM, Pandey SC (1975) Increased disaccharidase activity in human diabetics. Am J Clin Nutr 28(6):621–625. https://doi.org/10.1093/ajcn/28.6.621
Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ (2020) Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health 10(1):107. https://doi.org/10.2991/2Fjegh.k.191028.001
Ruiz J. (2012) Diabète [Diabetes mellitus]. Rev Med Suisse. 18;8(324):88-90. French.
Gillespie KM (2006) Type 1 diabetes: pathogenesis and prevention. CMAJ. 175(2):165–170. https://doi.org/10.1503/cmaj.060244
Kahn BB, Flier JS (2000 Aug) Obesity and insulin resistance. J Clin Invest 106(4):473–481. https://doi.org/10.1172/JCI10842
Wei JP, Wang QH, Zheng HJ, Wei F (2018) Research progress on non-drug treatment for blood glucose control of type 2 diabetes mellitus. Chin J Integr Med 24(10):723–727. https://doi.org/10.1007/s11655-018-2844-2
Baker KD, Thummel CS (2007) Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6(4):257–266. https://doi.org/10.1016/j.cmet.2007.09.002
Graham P, Pick L (2017) Drosophila as a model for diabetes and diseases of insulin resistance. Curr Top Dev Biol 121:397–419. https://doi.org/10.1016/bs.ctdb.2016.07.011
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Asp Med 34(2-3):121–138. https://doi.org/10.1016/j.mam.2012.07.001
Joost H-G, Thorens B (2001) The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol 18(4):247–256. https://doi.org/10.1080/09687680110090456
Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A (2015) Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem J 466(2):203–218. https://doi.org/10.1042/BJ20141384
Lizunov VA, Stenkula K, Troy A, Cushman SW, Zimmerberg J (2013) Insulin regulates Glut4 confinement in plasma membrane clusters in adipose cells. PLoS One 8(3):e57559. https://doi.org/10.1371/journal.pone.0057559
Tunduguru R, Thurmond DC (2017 Nov) Promoting glucose transporter-4 vesicle trafficking along cytoskeletal tracks: PAK-Ing them out. Front Endocrinol (Lausanne) 20(8):329. https://doi.org/10.3389/fendo.2017.00329
Khan MSH, Hegde V (2020) Obesity and diabetes mediated chronic inflammation: a potential biomarker in Alzheimer’s disease. J Pers Med 10(2):42. https://doi.org/10.3390/jpm10020042
Hom FG, Goodner CJ, Berrie MA (1984) A [3H]2-deoxyglucose method for comparing rates of glucose metabolism and insulin responses among rat tissues in vivo. Validation of the model and the absence of an insulin effect on the brain. Diabetes. 33(2):141–152. https://doi.org/10.2337/diab.33.2.141
Seaquist ER, Damberg GS, Tkac I, Gruetter R (2001) The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 50(10):2203–2209. https://doi.org/10.2337/diabetes.50.10.2203
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM (2005) Insulin and insulin-like growth factor expression and function deteriorate with the progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis 8(3):247–268. https://doi.org/10.3233/Jan-2005-8304
Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU (2002) Insulinresponsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res 107:157–165
Kroner Z (2009) The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Altern Med Rev 14:373–379
Hirvonen J, Virtanen KA, Nummenmaa L, Hannukainen JC, Honka MJ, Bucci M, Nesterov SV, Parkkola R, Rinne J, Iozzo P, Nuutila P (2011) Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 60:443–447
Cholerton B, Baker LD, Craft S (2013) Insulin, cognition, and dementia. Eur J Pharmacol 719:170–179
Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG (2012) An antidiabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Abeta oligomers. J Clin Invest 122:1339–1353
de la Monte SM, Tong M (2014) Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 88:548–559
Bergman O, Westberg L, Nilsson LG, Adolfsson R, Eriksson E (2010) Preliminary evidence that polymorphisms in dopamine-related transcription factors LMX1A, LMX1B and PITX3 are associated with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 34(6):1094–1097. https://doi.org/10.1016/j.pnpbp.2010.05.032
Zheng H, Koo EH (2011) Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener 6(1):27. https://doi.org/10.1186/1750-1326-6-27
Devi L, Alldred MJ, Ginsberg SD, Ohno M (2012) Mechanisms underlying insulin deficiency-induced acceleration of beta-amyloidosis in a mouse model of Alzheimer’s disease. PLoS One 7:e32792
Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R (2013) Insulin has multiple anti-amyloidogenic effects on human neuronal cells. Endocrinology 154:375–387
Watson GS, Craft S (2004) Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol 490(1-3):97–113. https://doi.org/10.1016/j.ejphar.2004.02.048
Kopf SR, Baratti CM (1996) Effects of posttraining administration of glucose on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem 65(3):253–260. https://doi.org/10.1006/nlme.1996.0030
Ragozzino ME, Unick KE, Gold PE (1996) Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A 93(10):4693–4698. https://doi.org/10.1073/pnas.93.10.4693
Qiu WQ, Folstein MF (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging 27:190–198
Haque R, Nazir A (2014) Insulin-degrading enzyme: a link between Alzheimer’s and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets 13:259–264
Kreneisz O, Chen X, Fridell YW, Mulkey DK (2010) Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport. 21:1116–1120
Kaneto H (2015) [Pathophysiology of type 2 diabetes mellitus]. Nihon Rinsho. 73(12):2003-7. Japanese.
Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14(10):591–604. https://doi.org/10.1038/s41574-018-0048-7
Redondo MT, Beltrán-Brotóns JL, Reales JM, Ballesteros S (2016) Executive functions in patients with Alzheimer’s disease, type 2 diabetes mellitus patients, and cognitively healthy older adults. Exp Gerontol 83:47–55. https://doi.org/10.1016/j.exger.2016.07.013
McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND (2016) Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 176(7):969–978. https://doi.org/10.1001/jamainternmed.2016.2275
Pilotto A, Noale M, Maggi S, Addante F, Tiengo A, Perin PC et al (2014) Hypoglycemia is independently associated with multidimensional impairment in elderly diabetic patients. Biomed Res Int 2014:1–7. https://doi.org/10.1155/2014/906103
Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J (1991) Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Phys 260(1 Pt 1):E67–E74. https://doi.org/10.1152/ajpendo.1991.260.1.E67
Baglietto-Vargas, David, Jessica Shi, Devin M. Yaeger, Ager, Rahasson, LaFerla, Frank M. (2016) Diabetes and Alzheimer’s disease crosstalk.Neuroscience and Biobehavioral Reviews.
Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM (2015) Hyperglycemia modulates extracellular amyloid-beta concentrations and neuronal activity in vivo. J Clin Invest 125:2463–2467
Srikanth V, Mazurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G (2011) Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging 32(5):763–777. https://doi.org/10.1016/j.neurobiolaging.2009.04.016
Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, Woodward M, Striker GE, Vlassara H (2014) Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A 111:4940–4945
Beeri MS, Moshier E, Schmeidler J, Godbold J, Uribarri J, Reddy S et al (2011) Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev 132(11-12):583–587. https://doi.org/10.1016/j.mad.2011.10.007
Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, Catalano MG, Smith MA, Perry G, Danni O, Boccuzzi G, Tabaton M (2012) AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging 33(196):e113–e127
Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, Park YM, Lee B (2012) Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer’s disease. PLoS One 7:e37917
Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS (2014) Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer’s disease. Exp Mol Med 46:e75
West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, Uribarri J, Vlassara H, Silverman JM, Beeri MS (2014) Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Aging Dev 140:10–12
Ugrankar R, Berglund E, Akdemir F, Tran C, Kim MS, Noh J, Schneider R, Ebert B, Graff JM (2015) Drosophila glucose screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nat Commun 21(6):7102. https://doi.org/10.1038/ncomms8102
Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12(15):1293–1300. https://doi.org/10.1016/s0960-9822(02)01043-6
Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 296(5570):1118–1120. https://doi.org/10.1126/science.1070058
Lee G, Park JH (2004) Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone- encoding gene in Drosophila melanogaster. Genetics. 167:311–323
Nayak N, Mishra M (2019) Simple techniques to study multifaceted diabesity in the fly model. Toxicol Mech Methods 1–29. https://doi.org/10.1080/15376516.2019.1634171
Wessells R, Fitzgerald E, Piazza N et al (2009) d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell 8(5):542–552. https://doi.org/10.1111/j.1474-9726.2009.00504.x
Teleman AA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425(1):13–26. https://doi.org/10.1042/BJ20091181
Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Droege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. ProcNatlAcadSci U S A 102(8):3105–3110. https://doi.org/10.1073/pnas.0405775102
Haselton A, EffatSharmin JS, MeghaSah PP, Fridell Y-WC (2010) Partial ablation of adult Drosophilainsulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle 9(15):3135–3143. https://doi.org/10.4161/cc.9.15.12458
Nishida Y, Hata M, Nishizuka Y, Rutter WJ, Ebina Y (1986) Cloning of a Drosophila cDNA encoding a polypeptide similar to the human insulin receptor precursor. BiochimBiophys Res Commun 141(2):474–481. https://doi.org/10.1016/s0006-291x(86)80197-8
Yamaguchi T, Fernandez R, Roth RA (1995) Comparison of the signaling abilities of the Drosophila and human insulin receptors in mammalian cells. Biochemistry. 34(15):4962–4968. https://doi.org/10.1021/bi00015a007
Inoue YH, Katsube H, Hinami Y (2018) Drosophila models to investigate insulin action and mechanisms underlying human diabetes mellitus. Adv Exp Med Biol 1076:235–256. https://doi.org/10.1007/978-981-13-0529-0_13
Kim SK, Rulifson EJ (2004a) Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiac cells. Nature. 431(7006):316–320. https://doi.org/10.1038/nature02897
Zhao X, Karpac J (2017) Muscle directs diurnal energy homeostasis through a myokine-dependent hormone module in Drosophila. Curr Biol 27(13):1941–1955.e6. https://doi.org/10.1016/j.cub.2017.06.004
Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4(6):842–849. https://doi.org/10.1242/dmm.007948
Yu S, Zhang G, Jin LH (2018) A high-sugar diet affects cellular and humoral immune responses in Drosophila. Exp Cell Res 368(2):215–224. https://doi.org/10.1016/j.yexcr.2018.04.032
Westfall S, Lomis N, Prakash S (2018) Longevity extension in Drosophila through gut-brain communication. Sci Rep 8(1):8362. https://doi.org/10.1038/s41598-018-25382-z
Chi W, Zhang L, Du W, Zhuang X (2014) A nutritional conditional lethal mutant due to pyridoxine 5’-phosphate oxidase deficiency in Drosophila melanogaster. G3 (Bethesda) 4(6):1147–1154. https://doi.org/10.1534/g3.114.011130
Mascolo E, Amoroso N, Saggio I, Merigliano C, Vernì F (2020) Pyridoxine/pyridoxamine 5’-phosphate oxidase (Sgll/PNPO) is important for DNA integrity and glucose homeostasis maintenance in Drosophila. J Cell Physiol 235(1):504–512. https://doi.org/10.1002/jcp.28990
Jeibmann A, Paulus W (2009) Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci 10(2):407–440. https://doi.org/10.3390/ijms10020407
Shulman JM, Chipendo P, Chibnik LB, Aubin C, Tran D, Keenan BT, Kramer PL, Schneider JA, Bennett DA, Feany MB, De Jager PL (2011) Functional screening of Alzheimer pathology genome-wide association signals in Drosophila. Am J Hum Genet 88:232–238
Wasco W, Bupp K, Magendantz M, Gusella JF, Tanzi RE, Solomon F (1992) Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta-protein precursor. Proc Natl Acad Sci U S A 89:10758–10762
Luo L, Tully T, White K (1992) Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 9(4):595–605. https://doi.org/10.1016/0896-6273(92)90024-8
Chakraborty R, Vepuri V, Mhatre SD, Paddock BE, Miller S et al (2011) Characterization of a Drosophila Alzheimer’s disease model: pharmacological rescue of cognitive defects. PLoS One 6:e20799
Iijima K, Chiang HC, Hearn SA, Hakker I, Gatt A, Shenton C, Granger L, Leung A, Iijima-Ando K, Zhong Y (2008) Abeta42 mutants with different aggregation profiles induce distinct pathologies in Drosophila. PLoS One 3(2):e1703. https://doi.org/10.1371/journal.pone.0001703
Williams DW, Tyrer M, Shepherd D (2000) Tau and tau reporters disrupt central projections of sensory neurons in Drosophila. J Comp Neurol 428(4):630–640. https://doi.org/10.1002/1096-9861(20001225)428:4<630::aid-cne4>3.0.co;2-x
Denes AS, Jékely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D (2007) Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129:277–288
Sivanantharajah L, Mudher A, Shepherd D (2019) An evaluation of Drosophila as a model system for studying tauopathies such as Alzheimer’s disease. J Neurosci Methods. https://doi.org/10.1016/j.jneumeth.2019.01.001
Nichols CD (2006) Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther 112(3):677–700. https://doi.org/10.1016/j.pharmthera.2006.05.012
Yu H, Saura CA, Choi S-Y, Sun LD, Yang X, Handler M et al (2001) APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron 31(5):713–726. https://doi.org/10.1016/s0896-6273(01)00417-2
Kashiwaya Y, Bergman C, Lee J-H, Wan R, King MT, Mughal MR et al (2013) A ketone ester diet exhibits anxiolytic and cognition-sparing properties and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging 34(6):1530–1539. https://doi.org/10.1016/j.neurobiolaging.2012.11.023
Hoppe JB, Salbego CG, Cimarosti H (2015) SUMOylation: a novel neuroprotective approach for Alzheimer’s disease? Aging Dis 6:322–330
Martins WC, Tasca CI, Cimarosti H (2016) Battling Alzheimer’s disease: targeting SUMOylation-mediated pathways. Neurochem Res 41(3):568–578. https://doi.org/10.1007/s11064-015-1681-3
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259:5301–5305
Pierluigi M, Di Domenico F, Butterfield DA (2015) mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 84:39
Meijer AJ, Lorin S, Blommaart EF, Codogno P (2014) Regulation of autophagy by amino acids and mTOR-dependent signal transduction. Amino Acids
Tsuda L, Lim Y-M (2018) Alzheimer’s disease model system using Drosophila. Drosophila Models for Human Diseases 25–40. https://doi.org/10.1007/978-981-13-0529-0_3
Alzheimer’s disease facts and figures: Carol J. Farran, D.N.Sc., R.N., F.A.A.N. (Caregiving section), Bryan D. James, Ph.D. (Prevalence and Mortality sections), Tricia J. Johnson, Ph.D. (Use and costs of healthcare, long-term care, and hospice section), Ken P. Scholz, Ph.D. (Special Report), and Jennifer Weuve, M.P.H., Sc.D. (Prevalence and Mortality sections), 2011 https://doi.org/10.1016/j.jalz.2011.02.004
Rubin GM, Yankell MD, Wortman JR, Gabor Miklos GL, Nelson CR et al (2000) Comparative genomics of the eukaryotes. Science. 287:2204–2215
Greeve I, Kretzschmar D, Tschäpe JA, Beyn A, Brellinger C, Schweizer M, Nitsch RM, Reifegerste R (2004) Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci 24(16):3899–3906. https://doi.org/10.1523/JNEUROSCI.0283-04.2004
Álvarez-Rendón JP, Salceda R, Riesgo-Escovar JR (2018a) Drosophila melanogaster as a model for diabetes type 2 progression. Biomed Res Int 2018:1–16. https://doi.org/10.1155/2018/1417528
Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6:e1000857. https://doi.org/10.1371/journal.pgen.1000857
Gáliková M, Diesner M, Klepsatel P, Hehlert P, Xu Y, Bickmeyer I, Predel R, Kühnlein RP (2015) Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics 201(2):665–683. https://doi.org/10.1534/genetics.115.178897
Philippidou P, Dasen JS (2013) Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80(1):12–34. https://doi.org/10.1016/j.neuron.2013.09.020
Álvarez-Rendón JP, Salceda R, Riesgo-Escovar JR (2018b) Drosophila melanogaster as a model for diabetes type 2 progression. Biomed Res Int 24:2018. https://doi.org/10.1155/2018/1417528
Pandey A, Saini S, Khatoon R, Sharma D, Narayan G, Kar CD (2016) Overexpression of hsp27 rescued neuronal cell death and reduction in life- and health-span in Drosophila melanogaster against prolonged exposure to dichlorvos. Mol Neurobiol 53(5):3179–3193. https://doi.org/10.1007/s12035-015-9221-3
Guzman-Martinez L, Maccioni RB, Farías GA, Fuentes P, Navarrete LP (2019) Biomarkers for Alzheimer’s disease. Curr Alzheimer Res 16(6):518–528. https://doi.org/10.2174/1567205016666190517121140
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014 Dec) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26(12):2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Acknowledgements
The authors wish to thank the Lovely Professional University for providing basic facilities and are also grateful to Shabnam Shabir for her assistance in the corrections.
Author information
Authors and Affiliations
Contributions
RC, SY, and MPS wrote the draft of the manuscript, and MPS edited and proofread the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chakrabarty, R., Yousuf, S. & Singh, M.P. Contributive Role of Hyperglycemia and Hypoglycemia Towards the Development of Alzheimer’s Disease. Mol Neurobiol 59, 4274–4291 (2022). https://doi.org/10.1007/s12035-022-02846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02846-y