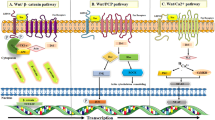
Abstract
The Janus-kinase (JAK) and signal transducer activator of transcription (STAT) signalling pathways regulate gene expression and control various factors involved in normal physiological functions such as cell proliferation, neuronal development, and cell survival. JAK activation phosphorylates STAT3 in astrocytes and microglia, and this phosphorylation has been linked to mitochondrial damage, apoptosis, neuroinflammation, reactive astrogliosis, and genetic mutations. As a regulator, peroxisome proliferator-activated receptor gamma (PPAR-gamma), in relation to JAK-STAT signalling, prevents this phosphorylation and aids in the treatment of the above-mentioned neurocomplications. Changes in cellular signalling may also contribute to the onset and progression of autism. Thus, PPAR-gamma agonist upregulation may be associated with JAK-STAT signal transduction downregulation. It may also be responsible for attenuating neuropathological changes by stimulating SOCS3 or involving RXR or SMRT, thereby reducing transcription of the various cytokine proteins and genes involved in neuronal damage. Along with JAK-STAT inhibitors, PPAR-gamma agonists could be used as target therapeutic interventions for autism. This research-based review explores the potential involvement and mutual regulation of JAK-STAT and PPAR-gamma signalling in controlling multiple pathological factors associated with autism.





Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- AIMs:
-
Abnormal involuntary movement
- Akt:
-
Protein kinase b
- ARID1:
-
AT-rich interaction domain 1B
- ASD:
-
ASD
- BBB:
-
Blood brain barrier
- BND:
-
Brain-derived neurotrophic factor
- BTBR:
-
BTBR T + Itpr3tf/J strain
- BTIC:
-
Brain tumour initiating cells
- CASP:
-
Caspase
- CAT:
-
Catalase
- CCL:
-
Chemokine receptor ligand
- CXCL:
-
Chemokine receptor
- DAT:
-
Dopamine transporter
- DC:
-
Dendritic cells
- DHA:
-
Docosahexaenoic acid
- EAE:
-
Experimental allergic encephalomyelitis
- EMF:
-
Electromagnetic fields
- ETC:
-
Electron transport chain
- Fmr1-KO:
-
Fragile X mental retardation 1 knockout
- FXS:
-
Fragile-x-syndrome
- GPx:
-
Glutathione peroxidase
- GFAP:
-
Glial fibrillary acidic protein
- H2O2:
-
Hydrogen peroxide
- ID:
-
Intellectual disabilities
- IDD:
-
Intellectual developmental disorders
- IL:
-
Interleukins
- INFg:
-
Interferon gamma
- iNOS:
-
Nitric oxide synthase
- JAK:
-
Janus kinase
- LEPR:
-
Leptin precursor receptor
- LPS:
-
Lipopolysaccharide
- LTD:
-
Long-term depression
- METH:
-
Methamphetamine
- MitoQ:
-
Mitochondria targeted antioxidant
- mtROS:
-
Mitochondrial reactive oxygen species
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NASH:
-
Non-alcoholic steatohepatitis
- NLN:
-
Neuroligin
- NMDAR:
-
N-methyl-D-aspartate receptor
- Nox oxidase:
-
NADPH oxidase
- NSC:
-
Neural stem cells
- OGD:
-
Oxygen glucose deprived
- OH:
-
Hydroxyl
- PD:
-
Parkinson disease
- PLC:
-
Phospholipase C
- PPARγ:
-
Peroxisome proliferation-activated receptor gamma
- ROS:
-
Reactive oxygen species
- RXR:
-
Retinoid-x-receptors
- SAE:
-
Sepsis-associated encephalopathy
- SERT:
-
Serotonin transporter
- SOD:
-
Superoxide dismutase
- SOCS:
-
Suppressor of cytokine signalling
- STAT:
-
Signal transducer and activator of transcription
- SVZ:
-
Subventricular zone
- TBI:
-
Traumatic brain injury
- TNF-α:
-
Tumour necrotic factor-alpha
- VPA:
-
Valproic acid
- X-ALD:
-
X-linked adrenoleukodystrophy
- ROS-R:
-
Reactive oxygen species-receptor
References
Bousoik Emira, Aliabadi Montazeri, Hamidreza (2018) “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front Oncol 8:287. https://doi.org/10.3389/fonc.2018.00287
Ahmad SF, Ansari MA, Nadeem A, Bakheet SA, Alzahrani MZ, Alshammari MA, Attia SM (2018) Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T+ Itpr3tf/J autistic mice. Eur J Pharmacol 829:70–78
Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH (2013) Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci 33(6):2481–2493. https://doi.org/10.1523/jneurosci.4440-12.2013
Ahmad, Sheikh F Ansari, Mushtaq A Nadeem, Ahmed; Bakheet, Saleh A.; AL-Ayadhi, Laila Y.; Attia, Sabry M. 2018. Elevated IL-16 expression is associated with development of immune dysfunction in children with autism. Psychopharmacology https://doi.org/10.1007/s00213-018-5120-4
Ahmad Sheikh F, Nadeem Ahmed, Ansari Mushtaq A, Bakheet Saleh A, Laila Yousef Al-Ayadhi, Attia Sabr M (2017) Upregulation of IL-9 and JAK-STAT signaling pathway in children with autism. Prog Neuro-Psychopharmacol Biol Psychiatry 79:472–480. https://doi.org/10.1016/j.pnpbp.2017.08.002
Nadeem Ahmed, Ahmad Sheikh F, Attia Sabry M, AL-Ayadhi Laila Y, Al-Harbi Naif O, Bakheet Saleh A (2020) Dysregulation in IL-6 receptors is associated with upregulated IL-17A related signaling in CD4+ T cells of children with autism. Prog Neuro-Psychopharmacol Biol Psychiatry 97:109783. https://doi.org/10.1016/j.pnpbp.2019.109783
Nadeem Ahmed, Ahmad Sheikh F, Attia Sabry M, AL-Ayadhi Laila Y, Bakheet Saleh A, Al-Harbi Naif O (2019) Oxidative and inflammatory mediators are upregulated in neutrophils of autistic children Role of IL-17A receptor signaling. Prog Neuro-Psychopharmacol Biol Psychiatry 90:204–211. https://doi.org/10.1016/j.pnpbp.2018.12.002
Napolitano M, Costa L, Palermo R, Giovenco A, Vacca A, Gulino A (2011) (2011) Protective effect of pioglitazone, a PPARγ ligand, in a 3 nitropropionic acid model of Huntington’s disease. Brain Res Bull 85(3–4):231–237. https://doi.org/10.1016/j.brainresbull.\
Morgenweck J, Griggs RB, Donahue RR, Zadina JE, Taylor BK (2013) PPARγ activation blocks development and reduces established neuropathic pain in rats. Neuropharmacology 70:236–46. https://doi.org/10.1016/j.neuropharm.2013.01.020
Domi E, Caputi FF, Romualdi P, Domi A, Scuppa G, Candeletti S, Ubaldi M (2019) Activation of PPARγ attenuates the expression of physical and affective nicotine withdrawal symptoms through mechanisms involving amygdala and hippocampus neurotransmission. J Neurosci 39(49):9864–9875
Meng QQ, Feng ZC, Zhang XL, Hu LQ, Wang M, Zhang HF, Li SM (2019) PPAR-γ activation exerts an anti-inflammatory effect by suppressing the NLRP3 inflammasome in spinal cord-derived neurons. Mediators Inflamm 2019:6386729. https://doi.org/10.1155/2019/6386729.PMID:31015796;PMCID:PMC6444263
Ahmad SF, Ansari MA, Nadeem A, Bakheet SA, Alsanea S, Al-Hosaini KA, Attia SM (2020) Inhibition of tyrosine kinase signaling by tyrphostin AG126 downregulates the IL-21/IL-21R and JAK/STAT pathway in the BTBR mouse model of autism. Neurotoxicology 77:1–11
Haim LB, Ceyzériat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, Guillermier M, Escartin C (2015) The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J Neurosci 35(6):2817–2829
Qin H, Buckley JA, Li X, Liu Y, Fox TH 3rd, Meares GP, Yu H, Yan Z, Harms AS, Li Y, Standaert DG, Benveniste EN (2016) Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci 36(18):5144–5159. https://doi.org/10.1523/JNEUROSCI.4658-15.2016
Canto E, Isobe N, Didonna A, Hauser SL, Oksenberg JR (2018) Aberrant STAT phosphorylation signaling in peripheral blood mononuclear cells from multiple sclerosis patients. J Neuroinflammation 15(1):1–11
Kumar N, Sharma N, Khera R, Gupta R, Mehan S (2021) Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab Brain Dis 36(5):911–925. https://doi.org/10.1007/s11011-021-00691-x
Mukthavaram R, Ouyang X, Saklecha R, Jiang P, Nomura N, Pingle SC, Kesari S (2015) Effect of the JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres. J Transl Med 13:1. https://doi.org/10.1186/s12967-015-0627-5
Hodges GE, Ménard C, Russo SJ (2016) Integrating interleukin-6 into depression diagnosis and treatment. Neurobiol Stress 4:15–22. https://doi.org/10.1016/j.ynstr.2016.03.003
McGregor G, Irving AJ, Harvey J (2017) Canonical JAK-STAT signaling is pivotal for long-term depression at adult hippocampal temporoammonic-CA1 synapses. FASEB J 31(8):3449–3466. https://doi.org/10.1096/fj.201601293rr
Xu Z Zhang Z Ma X Ping F Zheng X 2015 [Effect of PM2.5 on oxidative stress-JAK/STAT signaling pathway of human bronchial epithelial cells]. Wei Sheng Yan Jiu.;44 3 451–5. Chinese. PMID: 26137628.
Tiwari A, Khera R, Rahi S, Mehan S, Makeen HA, Khormi YH, Rehman MU, Khan A (2021) Neuroprotective effect of α-mangostin in the ameliorating propionic acid-induced experimental model of autism in wistar rats. Brain Sci 11(3):288. https://doi.org/10.3390/brainsci11030288.PMID:33669120;PMCID:PMC7996534
Rahi S. & Mehan S (2020). Understanding abnormal SMO-SHH signaling in autism spectrum disorder: potential drug target and therapeutic goals. Cell molecular neurobiolAdvance online publicationhttps://doi.org/10.1007/s10571-020-01010-1
Peters-Scheffer N, Didden R, Korzilius H, Sturmey P (2011) A meta-analytic study on the effectiveness of comprehensive ABA-based early intervention programs for children with Autism Spectrum Disorders. Res Autism Spectr Disord 5(1):60–69. https://doi.org/10.1016/j.rasd.2010.03.011
Jones EK, Hanley M, Riby DM (2020) Distraction, distress and diversity: exploring the impact of sensory processing differences on learning and school life for pupils with autism spectrum disorders. Res Autism Spectr Disord 72:101515
Chakrabarti S, Fombonne E (2001) Pervasive developmental disorders in preschool children. JAMA 285(24):3093–3099
Rahi S, Mehan S (2020). Understanding abnormal SMO-SHH signaling in autism spectrum disorder: potential drug target and therapeutic goals. Cell mol neurobiol Advance online publ 10.1007/s10571-020-01010-1
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520
Attia, SM.; Al-Hamamah, MA.; Ahmad, SF.; Nadeem, A.; Attia, MSM.; Ansari, MA.; Bakheet, SA.; Al-Ayadhi, LY. 2019. Evaluation of DNA repair efficiency in autistic children by molecular cytogenetic analysis and transcriptome profiling. DNA Repair, 102750 https://doi.org/10.1016/j.dnarep.2019.102750
Mehan S, Rahi S, Tiwari A, Kapoor T, Rajdev K, Sharma R, Khera H, Kosey S, Kukkar U, Dudi R (2020) Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen Res 15(6):1140–1149. https://doi.org/10.4103/1673-5374.270316
Gadow KD, DeVincent CJ, Pomeroy J, Azizian A (2004) Psychiatric symptoms in preschool children with PDD and clinic and comparison samples. J Autism Dev Disord 34(4):379–393
Lecavalier L (2006) Behavioural and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord 36(8):1101–1114. https://doi.org/10.1007/s10803-006-0147-5
Nadeem, Ahmed; Ahmad, Sheikh F.; Al-Harbi, Naif O.; AL-Ayadhi, Laila Y.; Attia, Sabry M.; Alasmari, Abdullah F; As Sobeai, Homood M; Bakheet, Saleh A. 2020. Ubiquitous plasticizer, Di-(2-ethylhexyl) phthalate enhances existing inflammatory profile in monocytes of children with autism. Toxicology, 152597 https://doi.org/10.1016/j.tox.2020.152597
Darnall JE, Kerr JIM, Stark GR (1994) Jak-Stat pathways and transcriptional activation in response to IFNs and other extra cellular signaling protein. Science 264:1415–1421
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53(1):409–435
Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, Mayfield RD, Harris RA (2016) Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep 6:27618. https://doi.org/10.1038/srep27618
Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR (2009) PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci 29(5):954–963. https://doi.org/10.1111/j.1460-9568.2009.06657.x
Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R (2013) Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res 37(8):1351–1360. https://doi.org/10.1111/acer.12091
Storer PD, Xu J, Chavis J, Drew PD (2005) Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol 161(1–2):113–122. https://doi.org/10.1016/j.jneuroim.2004.12.015
De-Fraja C, Conti L, Magrassi L, Govoni S, Cattaneo E (1998) Members of the JAK/STAT proteins are expressed and regulated during development in the mammalian forebrain. J Neurosci Res 54(3):320–330
Csabai D, Seress L, Varga Z, Ábrahám H, Miseta A, Wiborg O, Czéh B (2017) 2017 Electron microscopic analysis of hippocampal axo-somatic synapses in a chronic stress model for depression. Hippocampus 27(1):17–27. https://doi.org/10.1002/hipo.22650
Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK (2003) PPAR gamma and PPAR delta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A 100(11):6712–7. https://doi.org/10.1073/pnas.1031789100
Napimoga MH, Vieira SM, Dal-Secco D, Freitas A, Souto FO, Mestriner FL, Alves-Filho JC, Grespan R, Kawai T, Ferreira SH, Cunha FQ (2008) Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta 12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J Immunol 180(1):609–617. https://doi.org/10.4049/jimmunol.180.1.609
Harris SG, Phipps RP (2001) The nuclear receptor PPAR-gamma is expressed by mouse T lymphocytes and PPAR-gamma agonists induce apoptosis. Eur J Immunol 31(4):1098–1105. https://doi.org/10.1002/1521-4141(200104)31:4%3c1098::aid-immu1098%3e3.0.co;2-i
Padilla J, Leung E, Phipps RP (2002 Apr) Human B lymphocytes and B lymphomas express PPAR-gamma and are killed by PPAR-gamma agonists. Clin Immunol 103(1):22–33. https://doi.org/10.1006/clim.2001.5181
Garza JC, Guo M, Zhang W, Lu XY (2008) 2008 Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem 283(26):18238–47. https://doi.org/10.1074/jbc.M800053200
A Tiwari S Rahi S Mehan 2020 Elucidation of abnormal extracellular regulated kinase (ERK) signaling and associations with syndromic and non-syndromic autism Curr Drug Targets.Advanceonlinepublication.https://doi.org/10.2174/1389450121666201020155010
Singh RK, Jia C, Garcia F, Carrasco GA, Battaglia G, Muma NA (2010) Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J Psychopharmacol 24(7):1079–88. https://doi.org/10.1177/0269881109103090
Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB (2005) Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotox Res 8(3–4):295–304. https://doi.org/10.1007/BF03033983
Gu J, Li G, Sun T, Su Y, Zhang X, Shen J, Tian Z, Zhang J (2008) Blockage of the STAT3 signaling pathway with a decoy oligonucleotide suppresses growth of human malignant glioma cells. J Neurooncol 89(1):9–17. https://doi.org/10.1007/s11060-008-9590-9
Tsai MC, Chen WJ, Tsai MS, Ching CH, Chuang JI (2011) Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J Pineal Res 51(2):233–245. https://doi.org/10.1111/j.1600-079X.2011.00885.x
R Wafer P Tandon JEN Minchin 2017 The role of peroxisome proliferator-activated receptor gamma (PPARG) in adipogenesis: applying knowledge from the fish aquaculture industry to biomedical research Front Endocrinol (Lausanne) 8102https://doi.org/10.3389/fendo.2017.00102
Heming M, Gran S, Jauch SL, Fischer-Riepe L, Russo A, Klotz L, Hermann S, Schäfers M, Roth J, Barczyk-Kahlert K (2018) Peroxisome proliferator-activated receptor-γ modulates the response of macrophages to lipopolysaccharide and glucocorticoids. Front Immunol 9:893. https://doi.org/10.3389/fimmu.2018.00893
Jiang C, Ting AT, Seed B (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391(6662):82–86. https://doi.org/10.1038/34184
Abdullah Z, Geiger S, Nino-Castro A, Böttcher JP, Muraliv E, Gaidt M, Klotz L (2012) Lack of PPARγ in myeloid cells confers resistance to Listeria monocytogenes infection. PloS one 7(5):e37349
Abdullah Z, Geiger S, Nino-Castro A, Böttcher JP, Muraliv E, Gaidt M, Klotz L (2012) Lack of PPARγ in myeloid cells confers resistance to Listeria monocytogenes infection. PloS one 7(5):e37349
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Chinetti-Gbaguidi G (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6(2):137–143
Eslami H, Sharifi AM, Rahimi H, Rahati M (2014) Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci Lett 558:31–36
Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J (2005) The intracerebral application of the PPARγ-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci 22(1):278–282. https://doi.org/10.1111/j.1460-9568.2005.04200.x
Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J (2009) Neuronal PPAR-gamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci 29(19):6186–6195. https://doi.org/10.1523/JNEUROSCI.5857-08.2009
Combs CK, Bates P, Karlo JC, Landreth GE (2001) Regulation of β-amyloid stimulated pro-inflammatory responses by peroxisome proliferator-activated receptor α. Neurochem Int 39(5–6):449–457
Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK (2004) Ligands for the peroxisome proliferator-activated receptor-γ and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol 148(1–2):116–126
Yu JH, Kim KH, Kim H (2007) SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol 40(4):677–88. https://doi.org/10.1016/j.biocel.2007.10.007
Song EA, Lim JW, Kim H (2017) Docosahexaenoic acid inhibits IL-6 expression via PPARγ-mediated expression of catalase in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol 88:60–68. https://doi.org/10.1016/j.biocel.2017.05.011
Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang J-G, Baca M (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci 97(12):6493–6498. https://doi.org/10.1073/pnas.100135197
Ju KD, Lim JW, Kim H (2017) Peroxisome proliferator-activated receptor-gamma inhibits the activation of in cerulein-stimulated pancreatic acinar cells. J Cancer Prev 22(3):189–194. https://doi.org/10.15430/JCP.2017.22.3.189
Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li J (2005) Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res 46(9):1904–1913. https://doi.org/10.1194/jlr.m500033-jlr200
Natarajan C, Bright JJ (2002) Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun 3(2):59–70. https://doi.org/10.1038/sj.gene.6363832
Gulbins A, Grassmé H, Hoehn R, Kohnen M, Edwards MJ, Kornhuber J, Gulbins E (2016) Role of Janus-kinases in major depressive disorder. Neurosignals 2016 24(1):71–80. https://doi.org/10.1159/000442613
Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, Collett VJ, Hildebrandt L, Seaton G, Choi SL, Sim SE, Bradley C, Lee K, Zhuo M, Kaang BK, Gressens P, Dournaud P, Fitzjohn SM, Bortolotto ZA, Cho K, Collingridge GL (2012) The Jak/STAT pathway is involved in synaptic plasticity. Neuron 73(2):374–390. https://doi.org/10.1016/j.neuron
Wang X, Liu Q, Ihsan A, Huang L, Dai M, Hao H, Cheng G, Liu Z, Wang Y, Yuan Z (2012) JAK/STAT pathway plays a critical role in the pro-inflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicol Sci 127(2):412–24. https://doi.org/10.1093/toxsci/kfs106
Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY (1997) Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol 17(9):5328–5337
Charras A, Arvaniti P, Le Dantec C, Dalekos GN, Zachou K, Bordron A, Renaudineau Y (2019) JAK inhibitors and oxidative stress control. Front Immunol 10:2814
Wang XL, Qiao CM, Liu JO, Li CY (2017) Inhibition of the SOCS1-JAK2-STAT3 signaling pathway confers neuroprotection in rats with ischemic stroke. Cell Physiol Biochem 44(1):85–98. https://doi.org/10.1159/000484585
Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M (2007) Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol 36(6):361–365
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Erratum In Ann Neurol 57(1):67–81. https://doi.org/10.1002/ana.20315
Lee N, Jae Y, Kim M, Cho T, Lee C, Hong YR, Hyeon DY, Ahn S, Kwon H, Kim K, Jung JH, Chae S, Shin JO, Bok J, Byun Y, Hwang D, Koo J (2020) A pathogen-derived metabolite induces microglial activation via odorant receptors. FEBS J 287(17):3841–3870. https://doi.org/10.1111/febs.15234
Korbecki J, Bobiński R, Dutka M (2019) Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. https://doi.org/10.1007/s00011-019-01231-1
Millot P, San C, Bennana E, Porte B, Vignal N, Hugon J, Mouton-Liger F (2020) STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol Lett. https://doi.org/10.1016/j.imlet.2020.10.004
Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T (1993) IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260(5115):1808–1810. https://doi.org/10.1126/science.8511589
Schmidt S, Moric E, Schmidt M, Sastre M, Feinstein DL, Heneka MT (2004) Anti-inflammatory and antiproliferative actions of PPAR-gamma agonists on T lymphocytes derived from MS patients. J Leukoc Biol 75(3):478–485. https://doi.org/10.1189/jlb.0803402
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391(6662):79–82. https://doi.org/10.1038/34178
Khera R, Mehan S, Bhalla S, Kumar S, Alshammari A, Alharbi M, Sadhu SS (2022) Guggulsterone mediated JAK/STAT and PPAR-gamma modulation prevents neurobehavioral and neurochemical abnormalities in propionic acid-induced experimental model of autism. Molecules 27:889
Piochon C, Kano M, Hansel C (2016) LTD-like molecular pathways in developmental synaptic pruning. Nat Neurosci 19(10):1299–1310. https://doi.org/10.1038/nn.4389
Hansel C (2019) 2018 Deregulation of synaptic plasticity in autism. Neurosci Lett 1(688):58–61. https://doi.org/10.1016/j.neulet.2018.02.003
Huttenlocher PR (1990) Morphometric study of human cerebral cortex development. Neuropsychologia 28(6):517–527. https://doi.org/10.1016/0028-3932(90)90031-i
Farshbaf MJ, Ghaedi K, Shirani M, Nasr-Esfahani MH (2014) Peroxisome proliferator activated receptor gamma (PPARγ) as a therapeutic target for improvement of cognitive performance in Fragile-X. Med Hypotheses 82(3):291–294. https://doi.org/10.1016/j.mehy.2013.12.012
D’ Angelo M, Castelli V, Catanesi M, Antonosante A, Dominguez-Benot R, Ippoliti R, Cimini A (2019) PPARγ and cognitive performance. Int J Mol Sci 20(20):5068. https://doi.org/10.3390/ijms20205068
Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L (2009) Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem 42(10–11):1032–1040
Nadeem, Ahmed; Ahmad, Sheikh F.; Al-Harbi, Naif O.; Alasmari, Abdullah F.; AL-Ayadhi, Laila Y.; Alasmari, Fawaz; Ibrahim, Khalid E.; Attia, Sabry M.; Bakheet, Saleh A. 2020. Upregulation of enzymatic antioxidants in CD4+ T cells of autistic children. Biochimie, 171–172 205–212 https://doi.org/10.1016/j.biochi.2020.03.009
Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2(7) e134https://doi.org/10.1038/tp.2012.61
Nadeem, Ahmed; Ahmad, Sheikh F.; Attia, Sabry M.; Bakheet, Saleh A.; Al-Harbi, Naif O.; AL-Ayadhi, Laila Y. 2017. Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain, Behavior, and Immunity, S0889159117304257–. https://doi.org/10.1016/j.bbi.2017.09.010
Qu Y, Oyan AM, Liu R, Hua Y, Zhang J, Hovland R, Popa M, Liu X, Brokstad KA, Simon R, Molven A, Lin B, Zhang WD, McCormack E, Kalland KH, Ke XS (2013) Generation of prostate tumor-initiating cells is associated with elevation of reactive oxygen species and IL-6/STAT3 signaling. Cancer Res 73(23):7090–7100. https://doi.org/10.1158/0008-5472.CAN-13-1560
Waris G, Huh KW, Siddiqui A (2001) Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol 21(22):7721–7730. https://doi.org/10.1128/MCB.21.22.7721-7730.2001
Nadeem Ahmed, Ahmad Sheikh F, Attia Sabry M, AL-Ayadhi Laila Y, Al-Harbi Naif O, Bakheet Saleh A (2019) Dysregulated enzymatic antioxidant network in peripheral neutrophils and monocytes in children with autism. Prog Neuro-Psychopharmacology Biol Psychiatry 88:352–359. https://doi.org/10.1016/j.pnpbp.2018.08.020
Manea A, Tanase LI, Raicu M, Simionescu M (2010) Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. ArteriosclerThrombVasc Biol 30(1):105–112. https://doi.org/10.1161/ATVBAHA.109.193896
Al-Harbi Naif O, Ahmed Nadeem, Ahmad Sheikh F, AL-Ayadhi Laila Y, Al-Harbi Mohammad M, As Sobeai Homood M, Ibrahim Khalid E, Bakheet Saleh A (2020) Elevated expression of toll-like receptor 4 is associated with NADPH oxidase-induced oxidative stress in B cells of children with autism. Int Immunopharmacol 84:106555. https://doi.org/10.1016/j.intimp.2020.106555
Ahmed Nadeem, Ahmad Sheikh F, Bakheet Saleh A, Al-Harbi Naif O, AL-Ayadhi Laila Y, Attia Sabry M, Zoheir Khairy MA (2017) Toll-like receptor 4 signaling is associated with upregulated NADPH oxidase expression in peripheral T cells of children with autism. Brain Behav Immun 61:146–154. https://doi.org/10.1016/j.bbi.2016.12.024
Giampietro L, Gallorini M, De Filippis B, Amoroso R, Cataldi A, di Giacomo V (2019) PPAR-γ agonist GL516 reduces oxidative stress and apoptosis occurrence in a rat astrocyte cell line. Neurochem Int 126:239–245. https://doi.org/10.1016/j.neuint.2019.03.021
Chandra M, Miriyala S, Panchatcharam M (2017) PPARγand Its Role in Cardiovascular Diseases. PPAR Res 2017:1–10. https://doi.org/10.1155/2017/6404638
Randy LH, Guoying B (2007) Agonism of peroxisome proliferator receptor-gamma may have therapeutic potential for neuroinflammation and Parkinson’s disease. Curr Neuropharmacol 5(1):35–46. https://doi.org/10.2174/157015907780077123
Gupte AA, Liu JZ, Ren Y, Minze LJ, Wiles JR, Collins AR, Lyon CJ, Pratico D, Finegold MJ, Wong ST, Webb P, Baxter JD, Moore DD, Hsueh WA (2012) Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology 526:2001–11. https://doi.org/10.1002/hep.2394
Chiang MC, Chern Y, Huang RN (2012) PPARgamma rescue of the mitochondrial dysfunction in Huntington’s disease. Neurobiol Dis 45(1):322–328. https://doi.org/10.1016/j.nbd.2011.08.016
Gurney JG, McPheeters ML, Davis MM (2006) Parental report of health conditions and health care use among children with and without autism: national survey of children’s health. Arch PediatrAdolesc Med 160(8):825–830. https://doi.org/10.1001/archpedi.160.8.825
Albert, P. R., &Benkelfat, C. 2013 The neurobiology of depression—revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiat 67(5):446–457
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M (2015) Cumulative meta-analysis of interleukins 6 and 1β tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49:206–15. https://doi.org/10.1016/j.bbi.2015.06.001
Kong E, Sucic S, Monje FJ, Savalli G, Diao W, Khan D, Ronovsky M, Cabatic M, Koban F, Freissmuth M, Pollak DD (2015) STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behaviour. Erratum In Sci Rep 5:9009. https://doi.org/10.1038/srep09009
Guan X, Wang Q, Liu M, Sun A, Li X. 2020 Possible involvement of the IL-6/JAK2/STAT3 pathway in the hypothalamus in depressive-like behaviour of rats exposed to chronic mild stress. Neuropsychobiology.:1–9. https://doi.org/10.1159/000509908. Epub ahead of print.
Al-Samhari MM, Al-Rasheed NM, Al-Rejaie S, Al-Rasheed NM, Hasan IH, Mahmoud AM, Dzimiri N (2016) Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects. Exp Biol Med 241(5):509–518
Beheshti, F., Hosseini, M., Hashemzehi, M., Soukhtanloo, M., &Asghari, A. 2019 The effects of PPAR-γ agonist pioglitazone on anxiety and depression-like behaviours in lipopolysaccharide injected rats. Toxin Rev, 1–10.
Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, Calabrese JR (2014) PPAR-γ agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs 28(6):571–581. https://doi.org/10.1007/s40263-014-0158-2
Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, Chauhan V (2011) Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem 117(2):209–220
Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D, Barshop BA, Courchesne E, Haas RH (2000) Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol 15(6):357–361. https://doi.org/10.1177/088307380001500601
Abid H, Ryan ZC, Delmotte P, Sieck GC, Lanza IR (2020) Extramyocellular interleukin-6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J 34(11):14458–14472
Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S (2004) Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114(9):1281–1289. https://doi.org/10.1172/JCI21752
Rong JX, Klein JL, Qiu Y, Xie M, Johnson JH, Waters KM, Zhang V, Kashatus JA, Remlinger KS, Bing N, Crosby RM, Jackson TK, Witherspoon SM, Moore JT, Ryan TE, Neill SD, Strum JC (2011) Rosiglitazone induces mitochondrial biogenesis in differentiated murine 3T3-L1 and C3H/10T1/2 adipocytes. PPAR Res 2011:179454. https://doi.org/10.1155/2011/179454
El-Ansary, A., Zayed, N., Al-Ayadhi, L., Qasem, H., Anwar, M., Meguid, N. A.,&Bjørklund, G. 2019. GABA synaptopathy promotes the elevation of caspases 3 and 9 as pro-apoptotic markers in Egyptian patients with autism spectrum disorder. Acta NeurologicaBelgica, 1–13.
Eftekharian MM, Komaki A, Oskooie VK, Namvar A, Taheri M, Ghafouri-Fard S (2019) Assessment of apoptosis pathway in peripheral blood of autistic patients. J Mol Neurosci 69(4):588–596
Rozovski U, Harris DM, Li P, Liu Z, Wu JY, Grgurevic S, Faderl S, Ferrajoli A, Wierda WG, Martinez M, Verstovsek S, Keating MJ, Estrov Z (2016) At high levels, constitutively activated stat3 induces apoptosis of chronic lymphocytic leukemia cells. J Immunol 196(10):4400–9. https://doi.org/10.4049/jimmunol.1402108
Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Bronfman M (2007) Peroxisome proliferator-activated receptor γ up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem 282(51):37006–37015
Lin H, Lin TN, Cheung WM, Nian GM, Tseng PH, Chen SF, Chen JJ, Shyue SK, Liou JY, Wu CW, Wu (2002) KK Cyclooxygenase-1 and bicistronic cyclooxygenase-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation 105(16):1962–9. https://doi.org/10.1161/01.cir.0000015365.49180.05
Yang C, Jo SH, Csernus B, Hyjek E, Liu Y, Chadburn A, Wang YL (2007) Activation of peroxisome proliferator-activated receptor gamma contributes to the survival of T lymphoma cells by affecting cellular metabolism. Am J Pathol 170(2):722–732. https://doi.org/10.2353/ajpath.2007.060651
Anderson LT, Campbell M, Grega DM, Perry R, Small AM, Green WH (1984) Haloperidol in the treatment of infantile autism: effects on learning and behavioral symptoms. Am J Psychiatry 141(10):1195–1202. https://doi.org/10.1176/ajp.141.10.1195
Anderson LT, Campbell M, Adams P, Small AM, Perry R, Shell J (1989) The effects of haloperidol on discrimination learning and behavioural symptoms in autistic children. J Autism Dev Disord 19(2):227–239
Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, Flanagan SD (1991) The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA 266(13):1793–1800
Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N (2010) Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry 67(1):59–68. https://doi.org/10.1001/archgenpsychiatry.2009.137
Chao OY, Pathak SS, Zhang H, Dunaway N, Li JS, Mattern C, Yang YM (2020) Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol Brain 13(1):1–16
Berhow MT, Hiroi N, Kobierski LA, Hyman SE, Nestler EJ (1996) Influence of cocaine on the JAK–STAT pathway in the mesolimbic dopamine system. J Neurosci 16(24):8019–8026
Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IκBα induction and block of NFκB and iNOS activation. J Neurochem 88(2):494–501
Collin M, Thiemermann C (2003) The PPAR-γ ligand 15-deoxyΔ12, 14 prostaglandin J2 reduces the liver injury in endotoxic shock. Eur J Pharmacol 476(3):257–258
Collin M, Patel NS, Dugo L, Thiemermann C (2004) Role of peroxisome proliferator-activated receptor-γ in the protection afforded by 15-deoxyΔ12, 14 prostaglandin J2 against the multiple organ failure caused by endotoxin. Crit Care Med 32(3):826–831
Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ (2002) Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res 941(1–2):1–10. https://doi.org/10.1016/s0006-8993(02)02480-0
Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T (2003) Paris Autism Research International Sibpair Study Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34(1):27–29. https://doi.org/10.1038/ng1136
Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH Jr, Vicente A, Sommer SS (2005) Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry 10(4):329–332. https://doi.org/10.1038/sj.mp.4001629
Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Südhof TC (2011) Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci 108(33):13764–13769
D’Gama AM, Pochareddy S, Li M, Jamuar SS, Reiff RE, Lam ATN, Walsh CA (2015) Targeted DNA sequencing from autism spectrum disorder brains implicates multiple genetic mechanisms. Neuron 88(5):910–917
Nord AS, Roeb W, Dickel DE, Walsh T, Kusenda M, O’Connor KL, Malhotra D, McCarthy SE, Stray SM, Taylor SM, Sebat J (2011 Jun) STAART Psychopharmacology Network King B King MC McClellan JM 2011 Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. Eur J Hum Genet 19(6):727–31. https://doi.org/10.1038/ejhg.2011.24
Halgren C, Kjaergaard S, Bak M, Hansen C, El-Schich Z, Anderson CM, Henriksen KF, Hjalgrim H, Kirchhoff M, Bijlsma EK, Nielsen M, den Hollander NS, Ruivenkamp CA, Isidor B, Le Caignec C, Zannolli R, Mucciolo M, Renieri A, Mari F, Anderlid BM, Andrieux J, Dieux A, Tommerup N, Bache I (2012 Sep) 2011 Corpus callosum abnormalities, intellectual disability, speech impairment, and autism in patients with haploinsufficiency of ARID1B. Clin Genet 82(3):248–55. https://doi.org/10.1111/j.1399-0004.2011.01755.x
Santen GW, Aten E, Sun Y, Almomani R, Gilissen C, Nielsen M, Kant SG, Snoeck IN, Peeters EA, Hilhorst-Hofstee Y, Wessels MW, den Hollander NS, Ruivenkamp CA, van Ommen GJ, Breuning MH, den Dunnen JT, van Haeringen A, Kriek M (2012) Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet 44(4):379–380. https://doi.org/10.1038/ng.2217
Shibutani M, Horii T, Shoji H, Morita S, Kimura M, Terawaki N, Miyakawa T, Hatada I (2017) Arid1b haploinsufficiency causes abnormal brain gene expression and autism-related behaviours in mice. Int J Mol Sci 18(9):1872. https://doi.org/10.3390/ijms18091872
O’Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB (2014) Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS ONE 9(7):e102003. https://doi.org/10.1371/journal.pone.0102003
Chen E, Xu D, Lan X, Jia B, Sun L, C Zheng J, Peng H (2013) A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr Mol Med 13(9):1474–1484
Chen S, Dong Z, Cheng M, Zhao Y, Wang M, Sai N, Zhang X (2017) Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflammation 14(1):1–12
Chen XM, Yu YH, Wang L, Zhao XY, Li JR (2019) Effect of the JAK2/STAT3 signaling pathway on nerve cell apoptosis in rats with white matter injury. Eur Rev Med Pharmacol Sci 23(1):321–327
Duan W, Yang Y, Yi W, Yan J, Liang Z, Wang N, Yi D (2013) New Role of JAK2/STAT3 Signaling in endothelial cell oxidative stress injury and protective effect of melatonin. PLoS ONE 8(3):e57941. https://doi.org/10.1371/journal.pone.0057941
Wang B, Wang X, Yang S, Liu X, Feng D, Lu F, Gao G (2016) Neuroprotective effects of nitidine in Parkinson’s disease models through inhibiting microglia activation: role of the Jak2–Stat3 pathway. RSC Adv 6(75):71328–71337. https://doi.org/10.1039/c6ra11759g
Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, Halle A, Petzold GC (2019) Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol Med 11(2):e9665. https://doi.org/10.15252/emmm.201809665
Shibata N, Yamamoto T, Hiroi A, Omi Y, Kato Y, Kobayashi M (2009) Activation of STAT3 and inhibitory effects of pioglitazone on STAT3 activity in a mouse model of SOD1 mutated amyotrophic lateral sclerosis. Neuropathology 2010 Aug 30(4):353–360. https://doi.org/10.1111/j.1440-1789.2009.01078.x
Zhu YF, Wang WP, Zheng XF, Chen Z, Chen T, Huang ZY, Jia LJ, Lei WL (2020) Characteristic response of striatal astrocytes to dopamine depletion. Neural Regen Res 15(4):724–730. https://doi.org/10.4103/1673-5374.266917
Hao Y, Yang X, Chen C, Yuan-Wang, Wang X, Li M, Yu Z. (2010) STAT3 signalling pathway is involved in the activation of microglia induced by 2.45 GHz electromagnetic fields. Int J Radiat Biol.;86(1):27–36. https://doi.org/10.3109/09553000903264507. PMID: 20070213.
Sarmiento Soto, M., Larkin, J. R., Martin, C., Khrapitchev, A. A., Maczka, M., Economopoulos, V. Sibson, N. R. 2020. STAT3-mediated astrocyte reactivity associated with brain metastasis contributes to neurovascular dysfunction. Cancer Research, canres.2251.2020. https://doi.org/10.1158/0008-5472.can-20-2251
Chen, S. L., Cai, G. X., Ding, H. G., Liu, X. Q., Wang, Z. H., Jing, Y. W., ... & Wen, M. Y 2020 JAK/STAT signaling pathway-mediated microRNA-181b promoted blood-brain barrier impairment by targeting sphingosine-1-phosphate receptor 1 in septic rats. Annals Transl Med 8 21
Tan MSY, Sandanaraj E, Chong YK, Lim SW, Koh LWH, Ng WH, Tan NS, Tan P, Ang BT, Tang C (2019) A STAT3-based gene signature stratifies glioma patients for targeted therapy. Nat Commun 10(1):3601. https://doi.org/10.1038/s41467-019-11614-x
West AJ, Tsui V, Stylli SS, Nguyen HPT, Morokoff AP, Kaye AH, Luwor RB (2018) The role of interleukin-6-STAT3 signalling in glioblastoma. Oncol Lett 16:44095–4104. https://doi.org/10.3892/ol.2018.9227
McFarland BC, Ma JY, Langford CP, Gillespie GY, Yu H, Zheng Y, Nozell SE, Huszar D, Benveniste EN (2011) Therapeutic potential of AZD1480 for the treatment of human glioblastoma. Mol Cancer Ther 10(12):2384–93. https://doi.org/10.1158/1535-7163.MCT-11-0480
Träger U, Magnusson A, Lahiri Swales N, Wild E, North J, Lowdell M, Björkqvist M. 2013 ho JAK/STAT signalling in Huntington’s disease immune cells. PLoSCurr.Dec 13;5 https://doi.org/10.1371/currents.hd.5791c897b5c3bebeed93b1d1da0c0648
Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schäffer AA, Puck JM, Grimbacher B (2007) STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357(16):1608–1619. https://doi.org/10.1056/NEJMoa073687
Liu S, Liu X, Xiong H, Wang W, Liu Y, Yin L, Tu C, Wang H, Xiang X, Xu J, Duan B, Tao A, Zhao Z, Mei Z (2019) CXCL13/CXCR5 signaling contributes to diabetes-induced tactile allodynia via activating pERK, pSTAT3, pAKT pathways and pro-inflammatory cytokines production in the spinal cord of male mice. Brain Behav Immun 80:711–724. https://doi.org/10.1016/j.bbi.2019.05.020
Wang LH, Yang XY, Zhang X, Huang J, Hou J, Li J, Farrar WL (2004) Transcriptional inactivation of STAT3 by PPARγ suppresses IL-6-responsive multiple myeloma cells. Immunity 20(2):205–218
Guo T, Wang Y, Guo Y, Wu S, Chen W, Liu N, Wang Y, Geng D (2018) (2018) 1, 25–D3 Protects From Cerebral Ischemia by Maintaining BBB Permeability via PPAR-γ Activation. Front Cell Neurosci 17(12):480. https://doi.org/10.3389/fncel.2018.00480
Keledjian K, Tsymbalyuk O, Semick S, Moyer M, Negoita S, Kim K, Ivanova S, Gerzanich V, Simard JM (2020) The peroxisome proliferator-activated receptor gamma (PPARγ) agonist, rosiglitazone, ameliorates neurofunctional and neuroinflammatory abnormalities in a rat model of Gulf War Illness. PLoS ONE 15(11):e0242427. https://doi.org/10.1371/journal.pone.0242427
Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E (2011) Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 194:250–261. https://doi.org/10.1016/j.neuroscience.2011.07.046
Abd El-Abhar H, El Fattah MA, Wadie W, El-Tanbouly DM (2018) Cilostazol disrupts TLR-4 Akt/GSK-3β/CREB and IL-6/JAK-2/STAT-3/SOCS-3 crosstalk in a rat model of Huntington’s disease. Plos one 13(9):e0203837
Jensen KV, Cseh O, Aman A, Weiss S, Luchman HA (2017) The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 12(12):e0189670. https://doi.org/10.1371/journal.pone.0189670
Damm J, Harden LM, Gerstberger R, Roth J, Rummel C (2013) The putative JAK-STAT inhibitor AG490 exacerbates LPS-fever, reduces sickness behaviour, and alters the expression of pro-and anti-inflammatory genes in the rat brain. Neuropharmacology 71:98–111
Al DU, Ji TL, Yang B, Cao JF, Zhang XG, Li Y, Pan S, Zhang B, Hu ZB, Zeng XW (2013) Neuroprotective effect of AG490 in experimental traumatic brain injury of rats. Chin Med J (Engl) 126(15):2934–2937
Choi M, Kim H, Yang EJ, Kim HS (2020) Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J Pharmacol Sci 143(4):290–299
Leidgens V, Proske J, Rauer L, Moeckel S, Renner K, Bogdahn U, Riemenschneider MJ, Proescholdt M, Vollmann-Zwerenz A, Hau P, Seliger C (2017) Stattic and metformin inhibit brain tumor initiating cells by reducing STAT3-phosphorylation. Oncotarget 8(5):8250–8263. https://doi.org/10.18632/oncotarget.14159
Li SF, Ouyang BS, Zhao X, Wang YP. 2018 Analgesic effect of AG490, a Janus kinase inhibitor, on oxaliplatin-induced acute neuropathic pain. Neural Regen Res.;13(8):1471-1476. Erratum in: Neural Regen Res. 2019 Apr;14(4):612 https://doi.org/10.4103/1673-5374.235305
García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC (2005) Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry 57(8):885–894. https://doi.org/10.1016/j.biopsych.2005.01.007
García-Bueno B, Caso JR, Pérez-Nievas BG, Lorenzo P, Leza JC (2007) Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacol 32(6):1251–1260
Jahrling JB, Hernandez CM, Denner L, Dineley KT (2014) PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J Neurosci 34(11):4054–4063. https://doi.org/10.1523/JNEUROSCI.4024-13.2014
Martinez AA, Morgese MG, Pisanu A, Macheda T, Paquette MA, Seillier A, Cassano T, Carta AR, Giuffrida A (2015) Activation of PPAR-gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats. NeurobiolDis 74:295–304. https://doi.org/10.1016/j.nbd.2014.11.024
Chamberlain S, Gabriel H, Strittmatter W, Didsbury J (2020) An exploratory phase IIa Study of the PPAR delta/gamma agonist T3D–959 assessing metabolic and cognitive function in subjects with mild to moderate Alzheimer’s disease. J Alzheimer’s Disease 73(3):1085–1103
Kielian T, Syed MM, Liu S, Phulwani NK, Phillips N, Wagoner G, Drew PD, Esen N (2008) The synthetic peroxisome proliferator-activated receptor-gamma agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. J Immunol 180(7):5004–5016. https://doi.org/10.4049/jimmunol.180.7.5004
Seok H, Lee M, Shin E (2019) Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep 9:4414. https://doi.org/10.1038/s41598-019-40736-x
Mirza R, Sharma B. 2019 A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioural phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem
Z-J Liu Z-H Li L Liu W-X Tang Y Wang M-R Dong C Xiao 2016 Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease Front Pharmacol 7https://doi.org/10.3389/fphar.2016.00261
Weng Q-F, Chen G-B, Min-Guang Xu, Long R-T, Wang H, Wang X-Y, Jiang C-N, Yi X-N (2019) Upregulation of PPAR-gamma activity nhibits cyclooxygenase 2 expression in cortical neurons with N-methyl-d-aspartic acid induced excitatory neurotoxicity. Biotechnol Biotechnol Equip 33(1):1018–1023. https://doi.org/10.1080/13102818.2019.1634488
Katsouri L, Lim YM, Blondrath K, Eleftheriadou I, Lombardero L, Birch AM, Sastre M (2016) PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β-secretase in an Alzheimer’s disease model. Proc Natl Acad Sci 113(43):12292–12297. https://doi.org/10.1073/pnas.1606171113
Heneka MT, Klockgether T, Feinstein DL (2000) Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. Erratum In J Neurosci 2000 Nov 15 20(22) 20(18):6862–6867. https://doi.org/10.1523/JNEUROSCI.20-18-06862.2000
Wu XJ, Sun XH, Wang SW, Chen JL, Bi YH, Jiang DX (2018) Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR γ. Eur Rev Med Pharmacol Sci 22(17):5688–5696. https://doi.org/10.26355/eurrev_201809_15836
Bhandari R, Kuhad A (2017) Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem Int 103:8–23. https://doi.org/10.1016/j.neuint.2016.12.012
You, Y.-H. Qin Z.-Q., Zhang, H.-L. Yuan, Z.-H., & Yu X 2019 MicroRNA-153 promotes brain-derived neurotrophic factor and hippocampal neuron proliferation to alleviate autism symptoms through inhibition of JAK-STAT pathway by LEPR. Bioscience Reports BSR20181904 https://doi.org/10.1042/bsr20181904
Masciopinto F, Di Pietro N, Corona C, Bomba M, Pipino C, Curcio M, Sensi SL (2012) Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD and wild-type mice. Cell Death Dis 3(12):e448–e448. https://doi.org/10.1038/cddis.2012.189
Mirza R, Sharma B (2019) Benefits of Fenofibrate in prenatal valproic acid-induced autism spectrum disorder related phenotype in rats. Brain Res Bull 147:36–46. https://doi.org/10.1016/j.brainresbull.2019.02.003
Acknowledgements
The authors express their gratitude to Chairman, Mr. Parveen Garg, and Director, Dr. G. D. Gupta, ISF College of Pharmacy, Moga (Punjab), India, for their excellent vision and support.
Author information
Authors and Affiliations
Contributions
Writing—original draft of the review, Rishabh Khera and Sumit Kumar; Conceptualization, Supervision, Sidharth Mehan; Writing review & editing, literature survey, Pranshul Sethi, Sonalika Bhalla, Aradhana Prajapati.
All authors agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
Authors approve for submitting the publication.
Consent to Participate
Not applicable.
Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khera, R., Mehan, S., Kumar, S. et al. Role of JAK-STAT and PPAR-Gamma Signalling Modulators in the Prevention of Autism and Neurological Dysfunctions. Mol Neurobiol 59, 3888–3912 (2022). https://doi.org/10.1007/s12035-022-02819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02819-1