Abstract
Coincident excitation via different sensory modalities encoding objects of positive salience is known to facilitate learning and memory. With a view to dissect the contribution of visual cues in inducing adaptive neural changes, we monitored the lever press activity of a rat conditioned to self-administer sweet food pellets in the presence/absence of light cues. Application of light cues facilitated learning and consolidation of long-term memory. The superior colliculus (SC) of rats trained on light cue showed increased neuronal activity, dendritic branching, and brain-derived neurotrophic factor (BDNF) protein and mRNA expression. Concomitantly, the hippocampus showed augmented neurogenesis as well as BDNF protein and mRNA expression. While intra-SC administration of U0126 (inhibitor of ERK 1/2 and long-term memory) impaired memory formation, lidocaine (local anaesthetic) hindered memory recall. The light cue–dependent sweet food pellet self-administration was coupled with increased efflux of dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in the nucleus accumbens shell (AcbSh). In conditioned rats, pharmacological inhibition of glutamatergic signalling in dentate gyrus (DG) reduced lever press activity, as well as DA and DOPAC secretion in the AcbSh. We suggest that the neuroplastic changes in the SC and hippocampus might represent memory engrams sculpted by visual cues encoding reward information.







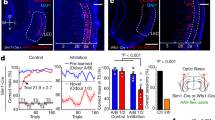
taken from the demarcated area (not to scale) in s and t (coordinates, bregma − 2.56 mm) (Paxinos and Watson, 1998). c, f, i, l, o and r show co-labeled cells with BrdU and NeuN antibodies and the insets (c’, f’, i’, l’, o’ and r’) show co-labeled cells at higher magnification respectively. The morphometric data were analysed using a one-way ANOVA followed by post hoc Bonferroni’s multiple comparison test and represented as mean ± SEM (n = 5/group) and power > 0.9. *p < 0.001 versus no reward + no light cue control; #p < 0.01, ##p < 0.001 versus no light cue control. Scale bar = 100 μm (a–r), and 25 μm (all insets). D3V, dorsal third ventricle; DG, dentate gyrus; str. or, stratum oriens; str. pyr, stratum pyramidale; str. rad, stratum radiatum

taken from the demarcated area (not to scale) in s and t (coordinates, bregma − 2.56 mm) (Paxinos and Watson, 1998). c, f, i, l, o and r show cells co-labeled with BrdU and NeuN antibodies, and the insets (c’, f’, i’, l’, o’ and r’) show co-labeled cells at higher magnification respectively. The morphometric data were analysed using a one-way ANOVA followed by post hoc Bonferroni’s multiple comparison test and represented as mean ± SEM (n = 5/group) and power > 0.9. *p < 0.001 versus no reward + no light cue control; #p < 0.001 versus no light cue control. Scale bar = 100 μm (a–r) and 25 μm (all insets). D3V, dorsal third ventricle; GCL, granule cell layer; ML, molecular layer; str. or, stratum oriens; str. pyr, stratum pyramidale; str. rad, stratum radiatum; SGZ, subgranular zone



Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- Acb:
-
Nucleus accumbens
- AcbSh:
-
Nucleus accumbens shell
- aCSF:
-
Artificial cerebrospinal fluid
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP:
-
Anterior posterior
- AUC:
-
Area under the curve
- BDNF:
-
Brain-derived neurotrophic factor
- BrdU:
-
5-bromo-2-deoxyuridine
- CREB:
-
cAMP response element-binding protein
- DA:
-
Dopamine
- DAB:
-
3,3′-Diaminobenzidine tetrahydrochloride
- DG:
-
Dentate gyrus
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- DpG:
-
Deep grey layer
- DpWh:
-
Deep white layer
- DV:
-
Dorsal ventral
- Elk-1:
-
Erythroblast transformation specific like-1
- ERK:
-
Extracellular signal-regulated kinase
- FR1:
-
Fixed ratio 1
- HPLC-ECD:
-
High-performance liquid chromatography with electrochemical detector
- HRP:
-
Horseradish peroxidase
- InG:
-
Intermediate grey layer
- InWh:
-
Intermediate white layer
- NMDA:
-
N-methyl-D-aspartic acid
- ip:
-
Intraperitoneal
- LTP:
-
Long-term potentiation
- MEK:
-
Mitogen-activated protein kinase
- ML:
-
Medial lateral
- NeuN:
-
Neuronal nuclear protein
- Op:
-
Optic layer
- PBS:
-
Phosphate-buffered saline
- PMSF:
-
Phenylmethylsulfonyl fluoride
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- RIPA:
-
Radioimmuno-precipitation assay
- RT:
-
Retention time
- SC:
-
Superior colliculus
- SDS:
-
Sodium dodecyl sulphate
- SuG:
-
Superficial grey layer
- VTA:
-
Ventral tegmental area
- Zo:
-
Zonal layer
References
Grabowska MJ, Jeans R, Steeves J, van Swinderen B (2020) Oscillations in the central brain of Drosophila are phase locked to attended visual features. Proc Natl Acad Sci USA 117(47):29925–29936. https://doi.org/10.1073/pnas.2010749117
Day JJ, Wheeler RA, Roitman MF, Carelli RM (2006) Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci 23(5):1341–1351. https://doi.org/10.1111/j.1460-9568.2006.04654.x
Day JJ, Jones JL, Carelli RM (2011) Nucleus accumbens neurons encode predicted and ongoing reward costs in rats. Eur J Neurosci 33(2):308–321. https://doi.org/10.1111/j.1460-9568.2010.07531.x
West EA, Carelli RM (2016) Nucleus accumbens core and shell differentially encode reward-associated cues after reinforcer devaluation. J Neurosci 36(4):1128–1139. https://doi.org/10.1523/JNEUROSCI.2976-15.2016
Ito S, Feldheim DA (2018) The mouse superior colliculus: an emerging model for studying circuit formation and function. Front Neural Circuits 12:10. https://doi.org/10.3389/fncir.2018.00010
Basso MA, May PJ (2017) Circuits for action and cognition: a view from the superior colliculus. Annu Rev Vis Sci 3:197–226. https://doi.org/10.1146/annurev-vision-102016-061234
Yu L, Stein BE, Rowland BA (2009) Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci 29(50):15910–15922. https://doi.org/10.1523/JNEUROSCI.4041-09.2009
Yu L, Xu J, Rowland BA, Stein BE (2016) Multisensory plasticity in superior colliculus neurons is mediated by association cortex. Cereb Cortex 26(3):1130–1137. https://doi.org/10.1093/cercor/bhu295
Lomber SG (2002) Learning to see the trees before the forest: reversible deactivation of the superior colliculus during learning of local and global visual features. Proc Natl Acad Sci USA 99(6):4049–4054. https://doi.org/10.1073/pnas.062551899
Krauzlis RJ, Lovejoy LP, Zénon A (2013) Superior colliculus and visual spatial attention. Annu Rev Neurosci 36:165–182. https://doi.org/10.1146/annurev-neuro-062012-170249
Crapse TB, Lau H, Basso MA (2018) A role for the superior colliculus in decision criteria. Neuron 97(1):181–194. https://doi.org/10.1016/j.neuron.2017.12.006
Drager UC, Hubel DH (1975) Physiology of visual cells in mouse superior colliculus and correlation with somatosensory and auditory input. Nature 253(5488):203–204. https://doi.org/10.1038/253203a0
Huerta MF, Harting JK (1983) Sublamination within the superficial gray layer of the squirrel monkey: an analysis of the tectopulvinar projection using anterograde and retrograde transport methods. Brain Res 261(1):119–126. https://doi.org/10.1016/0006-8993(83)91290-8
Hoy JL, Bishop HI, Niell CM (2019) Defined cell types in superior colliculus make distinct contributions to prey capture behavior in the mouse. Curr Biol 29(23):4130–4138. https://doi.org/10.1016/j.cub.2019.10.017
Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, Feldheim DA (2009) Retinal input instructs alignment of visual topographic maps. Cell 139(1):175–185. https://doi.org/10.1016/j.cell.2009.08.028
Lee AC, Yeung LK, Barense MD (2012) The hippocampus and visual perception. Front Hum Neurosci 6:91. https://doi.org/10.3389/fnhum.2012.00091
Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ (2003) Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron 37(3):473–484. https://doi.org/10.1016/s0896-6273(03)00030-8
Bender BN, Torregrossa MM (2020) Molecular and circuit mechanisms regulating cocaine memory. Cell Mol Life Sci 77(19):3745–3768. https://doi.org/10.1007/s00018-020-03498-8
Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A (2008) Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321(5896):1690–1692. https://doi.org/10.1126/science.1160873
Upadhya MA, Nakhate KT, Kokare DM, Singh U, Singru PS, Subhedar NK (2012) CART peptide in the nucleus accumbens shell acts downstream to dopamine and mediates the reward and reinforcement actions of morphine. Neuropharmacology 62(4):1823–1833. https://doi.org/10.1016/j.neuropharm.2011.12.004
Sangha S, Scheibenstock A, Morrow R, Lukowiak K (2003) Extinction requires new RNA and protein synthesis and the soma of the cell right pedal dorsal 1 in Lymnaea stagnalis. J Neurosci 23(30):9842–9851. https://doi.org/10.1523/JNEUROSCI.23-30-09842.2003
Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E, Tahir A, Sweatt JD (2013) DNA methylation regulates associative reward learning. Nat Neurosci 16(10):1445–1452. https://doi.org/10.1038/nn.3504
Dandekar MP, Singru PS, Kokare DM, Subhedar NK (2009) Cocaine- and amphetamine-regulated transcript peptide plays a role in the manifestation of depression: social isolation and olfactory bulbectomy models reveal unifying principles. Neuropsychopharmacology 34(5):1288–1300. https://doi.org/10.1038/npp.2008.201
Nakhate KT, Dandekar MP, Kokare DM, Subhedar NK (2009) Involvement of neuropeptide Y Y(1) receptors in the acute, chronic and withdrawal effects of nicotine on feeding and body weight in rats. Eur J Pharmacol 609(1–3):78–87. https://doi.org/10.1016/j.ejphar.2009.03.008
Kokare DM, Shelkar GP, Borkar CD, Nakhate KT, Subhedar NK (2011) A simple and inexpensive method to fabricate a cannula system for intracranial injections in rats and mice. J Pharmacol Toxicol Methods 64(3):246–250. https://doi.org/10.1016/j.vascn.2011.08.002
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, London. https://doi.org/10.1017/CBO9781107415324.004
Deehan GAJr, Engleman EA, Ding ZM, McBride WJ, Rodd ZA (2013) Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcohol Clin Exp Res 37(5):722–729. https://doi.org/10.1111/acer.12034
Kelley JB, Anderson KL, Altmann SL, Itzhak Y (2011) Long-term memory of visually cued fear conditioning: roles of the neuronal nitric oxide synthase gene and cyclic AMP response element-binding protein. Neuroscience 174:91–103. https://doi.org/10.1016/j.neuroscience.2010.11.005
Marshall-Phelps KLH, Riedel G, Wulff P, Woloszynowska-Fraser M (2020) Cerebellar molecular layer interneurons are dispensable for cued and contextual fear conditioning. Sci Rep 10(1):20000. https://doi.org/10.1038/s41598-020-76729-4
Zhong F, Liu L, Wei JL, Dai RP (2019) Step by step Golgi-cox staining for cryosection. Front Neuroanat 13:62. https://doi.org/10.3389/fnana.2019.00062
Sagarkar S, Choudhary AG, Balasubramanian N, Awathale SN, Somalwar AR, Pawar N, Kokare DM, Subhedar NK, Sakharkar AJ (2021) LSD1-BDNF activity in lateral hypothalamus-medial forebrain bundle area is essential for reward seeking behavior. Prog Neurobiol 202:102048. https://doi.org/10.1016/j.pneurobio.2021.102048
Harvey AR, Warton SS (1986) The morphology of neurons in rat tectal transplants as revealed by Golgi-Cox impregnation. Anat Embryol (Berl) 174(3):361–367. https://doi.org/10.1007/BF00698786
Kawade HM, Borkar CD, Shambharkar AS, Singh O, Singru PS, Subhedar NK, Kokare DM (2020) Intracellular mechanisms and behavioral changes in mouse model of attention deficit hyperactivity disorder: Importance of age-specific NMDA receptor blockade. Pharmacol Biochem Behav 188:172830. https://doi.org/10.1016/j.pbb.2019.172830
Sagarkar S, Bhamburkar T, Shelkar G, Choudhary A, Kokare DM, Sakharkar AJ (2017) Minimal traumatic brain injury causes persistent changes in DNA methylation at BDNF gene promoters in rat amygdala: a possible role in anxiety-like behaviors. Neurobiol Dis 106:101–109. https://doi.org/10.1016/j.nbd.2017.06.016
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Cameron HA, McKay RD (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435(4):406–417. https://doi.org/10.1002/cne.1040
Bekiari C, Giannakopoulou A, Siskos N, Grivas I, Tsingotjidou A, Michaloudi H, Papadopoulos GC (2015) Neurogenesis in the septal and temporal part of the adult rat dentate gyrus. Hippocampus 25(4):511–523. https://doi.org/10.1002/hipo.22388
Awathale SN, Dudhbhate BB, Rahangdale RR, Borkar CD, Subhedar NK, Kokare DM (2020) Denial of food to the hungry rat: a novel paradigm for induction and evaluation of anger-like emotion. J Neurosci Methods 341:108791. https://doi.org/10.1016/j.jneumeth.2020.108791
Bharne AP, Borkar CD, Bodakuntla S, Lahiri M, Subhedar NK, Kokare DM (2016) Pro-cognitive action of CART is mediated via ERK in the hippocampus. Hippocampus 26(10):1313–1327. https://doi.org/10.1002/hipo.22608
Wojtowicz JM, Kee N (2006) BrdU assay for neurogenesis in rodents. Nat Protoc 1(3):1399–1405. https://doi.org/10.1038/nprot.2006.224
Velazco-Cercas E, Beltran-Parrazal L, Morgado-Valle C, Lopez-Meraz ML (2020) Status epilepticus increases cell proliferation and neurogenesis in the developing rat cerebellum. Cerebellum 19(1):48–57. https://doi.org/10.1007/s12311-019-01078-6
Harte M, O’Connor WT (2005) Evidence for a selective prefrontal cortical GABA(B) receptor-mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience 130(1):215–222. https://doi.org/10.1016/j.neuroscience.2004.08.045
Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW (1998) Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience 82(4):1103–1114. https://doi.org/10.1016/s0306-4522(97)00366-7
Yang SC, Pan JT, Li HY (2004) CART peptide increases the mesolimbic dopaminergic neuronal activity: a microdialysis study. Eur J Pharmacol 494(2–3):179–182. https://doi.org/10.1016/j.ejphar.2004.05.018
Shahidani S, Reisi P, Naghdi N, Alaei H, Ramshini E (2012) Lesion of medial prefrontal cortex reduces morphine-induced extracellular dopamine level in the ventral tegmental area: a microdialysis study in rats. Pharmacol Biochem Behav 102(1):77–81. https://doi.org/10.1016/j.pbb.2012.03.009
Awathale SN, Choudhary AG, Subhedar NK, Kokare DM (2021) Neuropeptide CART modulates dopamine turnover in the nucleus accumbens: insights into the anatomy of rewarding circuits. J Neurochem 158(5):1172–1185. https://doi.org/10.1111/jnc.15479
Ramkumar K, Srikumar BN, Venkatasubramanian D, Siva R, Shankaranarayana Rao BS, Raju TR (2012) Reversal of stress-induced dendritic atrophy in the prefrontal cortex by intracranial self-stimulation. J Neural Transm (Vienna) 119(5):533–543. https://doi.org/10.1007/s00702-011-0740-4
Bowman RE, Hagedorn J, Madden E, Frankfurt M (2019) Effects of adolescent bisphenol-A exposure on memory and spine density in ovariectomized female rats: adolescence vs adulthood. Horm Behav 107:26–34. https://doi.org/10.1016/j.yhbeh.2018.11.004
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Acosta-Pena E, Camacho-Abrego I, Melgarejo-Gutierrez M, Flores G, Drucker-Colin R, Garcia-Garcia F (2015) Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse 69(1):15–25. https://doi.org/10.1002/syn.21779
Haller J, Toth M, Halasz J, De Boer SF (2006) Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol Behav 88(1–2):173–182. https://doi.org/10.1016/j.physbeh.2006.03.030
Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT, Hormann N, Chang WC, Zhang Z, Do JP, Yao S (2017) Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545(7655):477–481. https://doi.org/10.1038/nature22350
Bharne AP, Borkar CD, Subhedar NK, Kokare DM (2015) Differential expression of CART in feeding and reward circuits in binge eating rat model. Behav Brain Res 291:219–231. https://doi.org/10.1016/j.bbr.2015.05.030
Borkar CD, Bharne AP, Nagalakshmi B, Sakharkar AJ, Subhedar NK, Kokare DM (2018) Cocaine- and amphetamine-regulated transcript peptide (CART) alleviates MK-801-induced schizophrenic dementia-like symptoms. Neuroscience 375:94–107. https://doi.org/10.1016/j.neuroscience.2018.01.056
Labriola AR, Laemle LK (1977) Cellular morphology in the visual layer of the developing rat superior colliculus. Exp Neurol 55(1):247–268. https://doi.org/10.1016/0014-4886(77)90174-1
Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P (2003) A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci 6(9):974–980. https://doi.org/10.1038/nn1113
Crombag HS, Sutton JM, Takamiya K, Lee HK, Holland PC, Gallagher M, Huganir RL (2008) A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav Brain Res 191(2):178–183. https://doi.org/10.1016/j.bbr.2008.03.026
Matell MS, Della Valle RB (2017) Temporal specificity in Pavlovian-to-instrumental transfer. Learn Mem 25(1):8–20. https://doi.org/10.1101/lm.046383.117
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469(7328):53–57. https://doi.org/10.1038/nature09588
Maiya R, Mangieri RA, Morrisett RA, Heberlein U, Messing RO (2015) A selective role for Lmo4 in cue-reward learning. J Neurosci 35(26):9638–9647. https://doi.org/10.1523/JNEUROSCI.1740-15.2015
Schleyer M, Fendt M, Schuller S, Gerber B (2018) Associative learning of stimuli paired and unpaired with reinforcement: evaluating evidence from maggots, flies, bees, and rats. Front Psychol 9:1494. https://doi.org/10.3389/fpsyg.2018.01494
Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ (2004) Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79(3):439–450. https://doi.org/10.1016/j.pbb.2004.08.018
Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM (2015) Alpha-melanocyte stimulating hormone modulates ethanol self-administration in posterior ventral tegmental area through melanocortin-4 receptors. Addict Biol 20(2):302–315. https://doi.org/10.1111/adb.12126
Ramkumar K, Srikumar BN, Shankaranarayana Rao BS, Raju TR (2008) Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res 33(9):1651–1662. https://doi.org/10.1007/s11064-007-9511-x
Verpelli C, Piccoli G, Zibetti C, Zanchi A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E, Sala C (2010) Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci 30(17):5830–5842. https://doi.org/10.1523/JNEUROSCI.0119-10.2010
Elston GN, Fujita I (2014) Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology. Front Neuroanat 8:78. https://doi.org/10.3389/fnana.2014.00078
Schaefer ML, Wang M, Perez PJ, Coca Peralta W, Xu J, Johns RA (2019) Nitric oxide donor prevents neonatal isoflurane-induced impairments in synaptic plasticity and memory. Anesthesiology 130(2):247–262. https://doi.org/10.1097/ALN.0000000000002529
Doubell TP, Skaliora I, Baron J, King AJ (2003) Functional connectivity between the superficial and deeper layers of the superior colliculus: an anatomical substrate for sensorimotor integration. J Neurosci 23(16):6596–6607. https://doi.org/10.1523/JNEUROSCI.23-16-06596.2003
Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38(3):579–593. https://doi.org/10.1007/s10571-017-0510-4
Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM (2002) BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 114(3):795–805. https://doi.org/10.1016/s0306-4522(02)00301-9
Tongiorgi E (2008) Activity-dependent expression of brain-derived neurotrophic factor in dendrites: facts and open questions. Neurosci Res 61(4):335–346. https://doi.org/10.1016/j.neures.2008.04.013
Medina JH, Viola H (2018) ERK1/2: a key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci 11:361. https://doi.org/10.3389/fnmol.2018.00361
Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH (2008) BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA 105(7):2711–2716. https://doi.org/10.1073/pnas.0711863105
Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR (2002) Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22(5):1532–1540. https://doi.org/10.1523/JNEUROSCI.22-05-01532.2002
Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24(1):261–269. https://doi.org/10.1111/j.1460-9568.2006.04893.x
Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH (2007) Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron 53(2):261–277. https://doi.org/10.1016/j.neuron.2006.11.025
Day JJ (2008) Extracellular signal-related kinase activation during natural reward learning: a physiological role for phasic nucleus accumbens dopamine? J Neurosci 28(17):4295–4297. https://doi.org/10.1523/JNEUROSCI.0776-08.2008
Shiflett MW, Brown RA, Balleine BW (2010) Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci 30(8):2951–2959. https://doi.org/10.1523/JNEUROSCI.1778-09.2010
Faccidomo S, Salling MC, Galunas C, Hodge CW (2015) Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. Psychopharmacology 232(18):3417–3430. https://doi.org/10.1007/s00213-015-3993-z
Silingardi D, Angelucci A, De Pasquale R, Borsotti M, Squitieri G, Brambilla R, Putignano E, Pizzorusso T, Berardi N (2011) ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex. Front Behav Neurosci 5:84. https://doi.org/10.3389/fnbeh.2011.00084
Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA (2013) Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology 38(5):753–762. https://doi.org/10.1038/npp.2012.238
Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Abrous DN (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5(8):e214. https://doi.org/10.1371/journal.pbio.0050214
Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Piazza PV (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3(4):e1959. https://doi.org/10.1371/journal.pone.0001959
Rapanelli M, Frick LR, Zanutto BS (2011) Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS ONE 6(2):e14713. https://doi.org/10.1371/journal.pone.0014713
Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I (2009) Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods 59(2):100–107. https://doi.org/10.1016/j.vascn.2008.12.002
Nguemeni C, McDonald MW, Jeffers MS, Livingston-Thomas J, Lagace D, Corbett D (2018) Short- and long-term exposure to low and high dose running produce differential effects on hippocampal neurogenesis. Neuroscience 369:202–211. https://doi.org/10.1016/j.neuroscience.2017.11.026
Foreman N, Stevens R (1987) Relationships between the superior colliculus and hippocampus: neural and behavioral considerations. Behavioral and Brain Sciences 10:101–152. https://doi.org/10.1017/s0140525x00056521
Cooper BG, Miya DY, Mizumori SJ (1998) Superior colliculus and active navigation: role of visual and non-visual cues in controlling cellular representations of space. Hippocampus 8(4):340–372. https://doi.org/10.1002/(SICI)1098-1063(1998)8:4%3c340::AID-HIPO4%3e3.0.CO;2-L
Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ (2004) Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci USA 101(14):5064–5068. https://doi.org/10.1073/pnas.0308528101
Epp JR, Chow C, Galea LA (2013) Hippocampus-dependent learning influences hippocampal neurogenesis. Front Neurosci 7:57. https://doi.org/10.3389/fnins.2013.00057
Canales JJ (2007) Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci 257(5):261–270. https://doi.org/10.1007/s00406-007-0730-6
Bruel-Jungerman E, Rampon C, Laroche S (2007) Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci 18(2):93–114. https://doi.org/10.1515/revneuro.2007.18.2.93
Hayashi K, Kubo K, Kitazawa A, Nakajima K (2015) Cellular dynamics of neuronal migration in the hippocampus. Front Neurosci 9:135. https://doi.org/10.3389/fnins.2015.00135
Yau SY, Li A, So KF (2015) Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast 717958. https://doi.org/10.1155/2015/717958
McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P (2005) Subcortical loops through the basal ganglia. Trends Neurosci 28(8):401–407. https://doi.org/10.1016/j.tins.2005.06.006
Zhang GR, Zhao H, Choi EM, Svestka M, Wang X, Nagayach A, Singh A, Cook RG, Geller AI (2019) An identified ensemble within a neocortical circuit encodes essential information for genetically-enhanced visual shape learning. Hippocampus 29(8):710–725. https://doi.org/10.1002/hipo.23068
Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J (2004) High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J Neurosci Methods 140(1–2):163–168. https://doi.org/10.1016/j.jneumeth.2004.04.041
Kersante F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC (2013) A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol 591(10):2429–2441. https://doi.org/10.1113/jphysiol.2012.246298
Truchet B, Chaillan FA, Soumireu-Mourat B, Roman FS (2002) Learning and memory of cue-reward association meaning by modifications of synaptic efficacy in dentate gyrus and piriform cortex. Hippocampus 12(5):600–608. https://doi.org/10.1002/hipo.10097
Ben Mamou C, Gamache K, Nader K (2006) NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9(10):1237–1239. https://doi.org/10.1038/nn1778
Bast T, da Silva BM, Morris RG (2005) Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci 25(25):5845–5856. https://doi.org/10.1523/JNEUROSCI.0698-05.2005
Albensi BC (2007) The NMDA receptor/ion channel complex: a drug target for modulating synaptic plasticity and excitotoxicity. Curr Pharm Des 13(31):3185–3194. https://doi.org/10.2174/138161207782341321
Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297(5579):211–218. https://doi.org/10.1126/science.1071795
McKay S, Bengtson CP, Bading H, Wyllie DJ, Hardingham GE (2013) Recovery of NMDA receptor currents from MK-801 blockade is accelerated by Mg2+ and memantine under conditions of agonist exposure. Neuropharmacology 74:119–125. https://doi.org/10.1016/j.neuropharm.2013.01.024
Day M, Langston R, Morris RG (2003) Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424(6945):205–209. https://doi.org/10.1038/nature01769
Xu LS, Yang LX, Hu WW, Yu X, Ma L, Liu LY, Wei EQ, Chen Z (2005) Histamine ameliorates spatial memory deficits induced by MK-801 infusion into ventral hippocampus as evaluated by radial maze task in rats. Acta Pharmacol Sin 26(12):1448–1453. https://doi.org/10.1111/j.1745-7254.2005.00229.x
Iwamura E, Yamada K, Ichitani Y (2016) Involvement of hippocampal NMDA receptors in retrieval of spontaneous object recognition memory in rats. Behav Brain Res 307:92–99. https://doi.org/10.1016/j.bbr.2016.03.048
Talpos JC, Aerts N, Fellini L, Steckler T (2014) A touch-screen based paired-associates learning (PAL) task for the rat may provide a translatable pharmacological model of human cognitive impairment. Pharmacol Biochem Behav 122:97–106. https://doi.org/10.1016/j.pbb.2014.03.014
Zarrindast MR, Lashgari R, Rezayof A, Motamedi F, Nazari-Serenjeh F (2007) NMDA receptors of dorsal hippocampus are involved in the acquisition, but not in the expression of morphine-induced place preference. Eur J Pharmacol 568(1–3):192–198. https://doi.org/10.1016/j.ejphar.2007.04.015
Acknowledgements
SNA acknowledges the Indian Council of Medical Research (ICMR) for providing senior research fellowship (45/06/2020/PHA/BMS).This work was supported by grants from the Science and Engineering Research Board (SERB) (CRG/2020/004971), Govt. of India, New Delhi, India.AJS acknowledges the funds received from SERB (GOI; EMR/2017/000621) and Council for Scientific and Industrial Research [(CSIR), GOI (37[1718]/18/EMR-II)]. We thank Yash Bhatt, 6025 Falling View Lane, Cumming, GA 30040, USA, for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design, NKS, DMK, and SNA; behavioural, biochemical, and IHC data collection, SNA and AMW; protein and mRNA data collection, HMK, GJ, SS, and AJS; analysis and interpretation of results, SNA, AGC, NKS, AJS, and DMK; draft manuscript preparation, NKS, SNA, AGC, and DMK. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Protocols employed in the present study were carried out in accordance with the Institutional Animal Ethics Committee, Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, India.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Awathale, S.N., Waghade, A.M., Kawade, H.M. et al. Neuroplastic Changes in the Superior Colliculus and Hippocampus in Self-rewarding Paradigm: Importance of Visual Cues. Mol Neurobiol 59, 890–915 (2022). https://doi.org/10.1007/s12035-021-02597-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02597-2