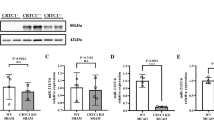
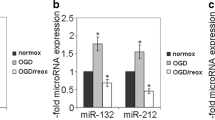
Abstract
Zika virus (ZIKV) is a neurotropic virus that causes microcephaly in newborns and Guillain-Barré syndrome (GBS) in adults. ZIKV is known to transmigrate through the blood–brain barrier (BBB) by utilizing different strategies. NS1 is a conserved flavivirus protein, which is secreted extracellularly. ZIKV-NS1 has been shown to target adherens junctions (AJs) and tight junctions (TJs) to disrupt the endothelial barrier integrity. The microRNAs are short non-coding RNAs, which post-transcriptionally regulate the gene expression by binding to 3’ UTR of the target gene. In the present study, we studied the ZIKV-NS1-mediated effect through hsa-miR-101-3p on the junctional barrier integrity in human brain microvascular endothelial cells. We exposed hBMVECs and hCMEC/D3 cells with ZIKV-NS1 at different time points (12 h and 24 h) with the doses 500 ng/mL and 1000 ng/mL. The change in the expression of VE-cadherin and claudin-5 was quantified using immunoblotting. The expression of the hsa-miR-101-3p was quantified using qRT-PCR. To prove the targeting of hsa-miR-101-3p to VE-cadherin, we transfected hsa-miR-101-3p mimic, scramble, hsa-miR-101-3p inhibitor, and Cy3 in the ZIKV-NS1-exposed hCMEC/D3 cells. The distribution and expression of the VE-cadherin and claudin-5 were observed using immunofluorescence and immunoblotting. The ZIKV-NS1 compromises the endothelial barrier integrity by disrupting the VE-cadherin and claudin-5 protein expression via hsa-miR-101-3p. The findings of this study suggest that ZIKV-NS1 dysregulates the adherens junction and tight junction proteins through hsa-miR-101-3p, which compromises the barrier integrity of human brain microvascular endothelial cells.







Similar content being viewed by others
Data Availability
All data generated in this study are available from the corresponding author on reasonable request.
Abbreviations
- ZIKV:
-
Zika virus
- RNA:
-
Ribonucleic acid
- miRNA:
-
MicroRNA
- NS1:
-
Non-structural protein 1
- hBMVECSs:
-
Primary human brain microvascular endothelial cells
- hCMEC/D3:
-
Human brain microvascular endothelial cell line
- BBB:
-
Blood–brain barrier
- VE-cadherin:
-
Vascular endothelial cadherin
- FBS:
-
Fetal bovine serum
- JEV:
-
Japanese encephalitis virus
- WNV:
-
West Nile virus
- DENV:
-
Dengue virus
- YFV:
-
Yellow fever virus
- TBEV:
-
Tick-borne encephalitis virus
References
Bhardwaj U, Pandey N, Rastogi M, Singh SK (2021) Gist of Zika virus pathogenesis. Virology 560:86–95. https://doi.org/10.1016/j.virol.2021.04.008
Dick GWAWA, Kitchen SF, Haddow AJ (1952) Zika Virus (I). Isolations and serological specificity. Trans Royal Soc Tropical Med Hygiene 46(5):509–520. https://doi.org/10.1016/0035-9203(52)90042-4
Pierson TC, Diamond MS (2020) The continued threat of emerging flaviviruses. Nat Microbiol 5(6):796–812. https://doi.org/10.1038/s41564-020-0714-0
Rastogi M, Sharma N, Singh SK (2016) Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J 13:131. https://doi.org/10.1186/s12985-016-0590-7
Alcon-LePoder S, Sivard P, Drouet MT, Talarmin A, Rice C, Flamand M (2006) Secretion of flaviviral non-structural protein NS1 from diagnosis to pathogenesis. Novartis Found Symp 277:233–47. https://doi.org/10.1002/0470058005.ch17 (discussion 247-53)
Young PR, Hilditch PA, Bletchly C, Halloran W (2000) An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol 38(3):1053–1057. https://doi.org/10.1128/JCM.38.3.1053-1057.2000
Macdonald J, Tonry J, Hall RA, Williams B, Palacios G, Ashok MS, Jabado O, Clark D et al (2005) NS1 protein secretion during the acute phase of West Nile virus infection. J Virol 79(22):13924–13933. https://doi.org/10.1128/JVI.79.22.13924-13933.2005
Puerta-Guardo H, Glasner DR, Espinosa DA, Biering SB, Patana M, Ratnasiri K, Wang C, Beatty PR et al (2019) Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep 26(6):1598-1613.e8. https://doi.org/10.1016/j.celrep.2019.01.036
Wang C, Puerta-Guardo H, Biering SB, Glasner DR, Tran EB, Patana M, Gomberg TA, Malvar C et al (2019) Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog 15(7):e1007938. https://doi.org/10.1371/journal.ppat.1007938
Rastogi M, Singh SK (2020) Zika virus NS1 affects the junctional integrity of human brain microvascular endothelial cells. Biochimie 176:52–61. https://doi.org/10.1016/j.biochi.2020.06.011
Gavard J (2013) Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr 7(6):455–461. https://doi.org/10.4161/cam.27330
Gavard J, Gutkind JS (2008) VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol 10(8):883–885. https://doi.org/10.1038/ncb0808-883
Singh SK, Pal Bhadra M, Girschick HJ, Bhadra U (2008) MicroRNAs--micro in size but macro in function. FEBS J 275(20):4929–44. https://doi.org/10.1111/j.1742-4658.2008.06624.x
Wei X, Xiang T, Ren G, Tan C, Liu R, Xu X, Wu Z (2013) miR-101 is down-regulated by the hepatitis B virus x protein and induces aberrant DNA methylation by targeting DNA methyltransferase 3A. Cell Signal 25(2):439–446. https://doi.org/10.1016/j.cellsig.2012.10.013
Sharma S, Chatterjee A, Kumar P, Lal S, Kondabagil K (2020) Upregulation of miR-101 during Influenza A virus infection abrogates viral life cycle by targeting mTOR pathway. Viruses 12(4):444. https://doi.org/10.3390/v12040444
Wang FZ, Weber F, Croce C, Liu CG, Liao X, Pellett PE (2008) Human cytomegalovirus infection alters the expression of cellular microRNA species that affect its replication. J Virol 82(18):9065–9074. https://doi.org/10.1128/JVI.00961-08
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H et al (2017) Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res 27(7):882–897. https://doi.org/10.1038/cr.2017.62
Song J, Hu Y, Li H, Huang X, Zheng H, Hu Y, Wang J, Jiang X et al (2018) miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerg Microbes Infect 7(1):155. https://doi.org/10.1038/s41426-018-0157-3
Burek M, Konig A, Lang M, Fiedler J, Oerter S, Roewer N, Bohnert M, Thal SC et al (2019) Hypoxia-induced microRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res 10(6):672–683. https://doi.org/10.1007/s12975-018-0683-2
Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, Scalzo F, He Z (2018) miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem 293(52):20041–20050. https://doi.org/10.1074/jbc.RA118.001858
Li D, Liu Y, Pei C, Zhang P, Pan L, Xiao J, Meng S, Yuan Z, Bi X (2017) miR-285-Yki/Mask double-negative feedback loop mediates blood-brain barrier integrity in Drosophila. Proc Natl Acad Sci U S A 114(12):E2365–E2374. https://doi.org/10.1073/pnas.1613233114
Ma Q, Dasgupta C, Li Y, Huang L, Zhang L (2017) MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int J Mol Sci 18(7):1356. https://doi.org/10.3390/ijms18071356
Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW et al (2018) MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J 32(2):935–944. https://doi.org/10.1096/fj.201700139RRR
Cerutti C, Soblechero-Martin P, Wu D, Lopez-Ramirez MA, de Vries H, Sharrack B, Male DK, Romero IA (2016) MicroRNA-155 contributes to shear-resistant leukocyte adhesion to human brain endothelium in vitro. Fluids Barriers CNS 13(1):8. https://doi.org/10.1186/s12987-016-0032-3
Bai Y, Zhang Y, Hua J, Yang X, Zhang X, Duan M, Zhu X, Huang W et al (2016) Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse. Sci Rep 6:35642. https://doi.org/10.1038/srep35642
Mishra R, Singh SK (2013) HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. J Neurosci 33(14):5992–6000. https://doi.org/10.1523/JNEUROSCI.4796-12.2013
Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C et al (2008) Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10(8):923–934. https://doi.org/10.1038/ncb1752
Greene C, Hanley N, Campbell M (2019) Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS 16(1):3. https://doi.org/10.1186/s12987-019-0123-z
Gresch O, Altrogge L (2012) Transfection of difficult-to-transfect primary mammalian cells. Methods Mol Biol 801:65–74. https://doi.org/10.1007/978-1-61779-352-3_5
Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK (2002) Efficient transfection method for primary cells. Tissue Eng 8(2):235–245. https://doi.org/10.1089/107632702753725003
Alimonti JB, Ribecco-Lutkiewicz M, Sodja C, Jezierski A, Stanimirovic DB, Liu Q, Haqqani AS, Conlan W et al (2018) Zika virus crosses an in vitro human blood brain barrier model. Fluids Barriers CNS 15(1):15. https://doi.org/10.1186/s12987-018-0100-y
Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y et al (2016) Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19(5):705–712. https://doi.org/10.1016/j.chom.2016.03.008
Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, Yoo JS, Ge J et al (2017) Asian Zika virus strains target CD14(+) blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol 2(11):1558–1570. https://doi.org/10.1038/s41564-017-0016-3
Hui L, Nie Y, Li S, Guo M, Yang W, Huang R, Chen J, Liu Y et al (2020) Matrix metalloproteinase 9 facilitates Zika virus invasion of the testis by modulating the integrity of the blood-testis barrier. PLoS Pathog 16(4):e1008509. https://doi.org/10.1371/journal.ppat.1008509
Jaeger AS, Murrieta RA, Goren LR, Crooks CM, Moriarty RV, Weiler AM, Rybarczyk S et al (2019) Zika viruses of African and Asian lineages cause fetal harm in a mouse model of vertical transmission. PLoS Negl Trop Dis 13(4):e0007343. https://doi.org/10.1371/journal.pntd.0007343
Jurado KA, Iwasaki A (2017) Zika virus targets blood monocytes. Nat Microbiol 2:1460–1461. https://doi.org/10.1038/s41564-017-0049-7
Khaiboullina S, Uppal T, Kletenkov K, St Jeor SC, Garanina E, Rizvanov A, Verma SC (2019) Transcriptome profiling reveals pro-inflammatory cytokines and matrix metalloproteinase activation in Zika virus infected human umbilical vein endothelial cells. Front Pharmacol 10: p. 642. https://doi.org/10.3389/fphar.2019.00642
Khaiboullina SF, Uppal T, Sarkar R, Gorzalski A, St Jeor S, Verma SC (2017) ZIKV infection regulates inflammasomes pathway for replication in monocytes. Sci Rep 7(1):16050. https://doi.org/10.1038/s41598-017-16072-3
Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E (2017) CD14(+)CD16(+) monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2(11):1462–1470. https://doi.org/10.1038/s41564-017-0035-0
Siemann DN, Strange DP, Maharaj PN, Shi PY, Verma S (2017) Zika virus infects human Sertoli cells and modulates the integrity of the in vitro blood-testis barrier model. J Virol 91(22):e00623–17. https://doi.org/10.1128/JVI.00623-17
Tsetsarkin KA, Acklin JA, Liu G, Kenney H, Teterina NL, Pletnev AG, Lim JK (2020) Zika virus tropism during early infection of the testicular interstitium and its role in viral pathogenesis in the testes. PLoS Pathog 16(7):e1008601. https://doi.org/10.1371/journal.ppat.1008601
Xu P, Shan C, Dunn TJ, Xie X, Xia H, Gao J, Allende Labastida J, Zou J et al (2020) Role of microglia in the dissemination of Zika virus from mother to fetal brain. PLoS Negl Trop Dis 14(7):0008413. https://doi.org/10.1371/journal.pntd.0008413
Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N et al (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534(7606):267–71. https://doi.org/10.1038/nature18296
Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA et al (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374(22):2142–2151. https://doi.org/10.1056/NEJMoa1601824
Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G et al (2016) Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388(10047):898–904. https://doi.org/10.1016/S0140-6736(16)30883-2
Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, ResmanRus K et al (2016) Zika virus associated with microcephaly. N Engl J Med 374(10):951–8. https://doi.org/10.1056/NEJMoa1600651
Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO et al (2016) Zika virus disrupts phospho-TBK1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep 16(10):2576–2592. https://doi.org/10.1016/j.celrep.2016.08.038
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R et al (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–8. https://doi.org/10.1126/science.aaf6116
Rosa-Fernandes L, Cugola FR, Russo FB, Kawahara R, de Melo Freire CC, Leite PEC, Bassi Stern AC, Angeli CB et al (2019) Zika virus impairs neurogenesis and synaptogenesis pathways in human neural stem cells and neurons. Front Cell Neurosci 13:64. https://doi.org/10.3389/fncel.2019.00064
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J et al (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18(5):587–590. https://doi.org/10.1016/j.stem.2016.02.016
Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19(2):258–265. https://doi.org/10.1016/j.stem.2016.04.014
Olmo IG, Carvalho TG, Costa VV, Alves-Silva J, Ferrari CZ, Izidoro-Toledo TC, da Silva JF, Teixeira AL et al (2017) Zika virus promotes neuronal cell death in a non-cell autonomous manner by triggering the release of neurotoxic factors. Front Immunol 8:1016. https://doi.org/10.3389/fimmu.2017.01016
Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Charpentier T et al (2017) Axl Mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep 18(2):324–333. https://doi.org/10.1016/j.celrep.2016.12.045
Hilgenfeld R (2016) Zika virus NS1, a pathogenicity factor with many faces. EMBO J 35(24):2631–2633. https://doi.org/10.15252/embj.201695871
Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E (2015) Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7(304):304ra141. https://doi.org/10.1126/scitranslmed.aaa3787
Lecuyer MA, Kebir H, Prat A (2016) Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim Biophys Acta 1862(3):472–482. https://doi.org/10.1016/j.bbadis.2015.10.004
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. https://doi.org/10.1038/nrn1824
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a020412. https://doi.org/10.1101/cshperspect.a020412
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W et al (1996) Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 87(2):630–641. https://doi.org/10.1182/blood.V87.2.630.bloodjournal872630
Kim JH, Hossain FM, Patil AM, Choi JY, Kim SB, Uyangaa E, Park SY, Lee JH et al (2016) Ablation of CD11c(hi) dendritic cells exacerbates Japanese encephalitis by regulating blood-brain barrier permeability and altering tight junction/adhesion molecules. Comp Immunol Microbiol Infect Dis 48:22–32. https://doi.org/10.1016/j.cimid.2016.07.007
Velandia-Romero ML, Calderon-Pelaez MA, Castellanos JE (2016) In vitro infection with dengue virus induces changes in the structure and function of the mouse brain endothelium. PLoS ONE 11(6):e0157786. https://doi.org/10.1371/journal.pone.0157786
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. https://doi.org/10.1083/jcb.200302070
Andras IE, Leda A, Contreras MG, Bertrand L, Park M, Skowronska M, Toborek M (2017) Extracellular vesicles of the blood-brain barrier: role in the HIV-1 associated amyloid beta pathology. Mol Cell Neurosci 79:12–22. https://doi.org/10.1016/j.mcn.2016.12.006
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A 106(6):1977–1982. https://doi.org/10.1073/pnas.0808698106
Mishra R, Singh SK (2014) HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci 15:80. https://doi.org/10.1186/1471-2202-15-80
Alpuche-Lazcano SP, McCullogh CR, Corpo OD, Rance E, Scarborough RJ, Mouland AJ, Sagan SM, Teixeira MM, Gatignol A (2018) Higher cytopathic effects of a Zika virus Brazilian isolate from Bahia compared to a Canadian-imported Thai strain. Viruses 10(2):53. https://doi.org/10.3390/v10020053
Monel B, Compton AA, Bruel T, Amraoui S, Burlaud-Gaillard J, Roy N, Guivel-Benhassine F, Porrot F et al (2017) Zika virus induces massive cytoplasmic vacuolization and paraptosis-like death in infected cells. EMBO J 36(12):1653–1668. https://doi.org/10.15252/embj.201695597
Chen J, Yang YF, Chen J, Zhou X, Dong Z, Chen T, Yang Y, Zou P et al (2017) Zika virus infects renal proximal tubular epithelial cells with prolonged persistency and cytopathic effects. Emerg Microbes Infect 6(8):e77. https://doi.org/10.1038/emi.2017.67
Ghezzi S, Cooper L, Rubio A, Pagani I, Capobianchi MR, Ippolito G, Pelletier J, Meneghetti MCZ et al (2017) Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res 140:13–17. https://doi.org/10.1016/j.antiviral.2016.12.023
Chen Q, Li X, Kong L, Xu Q, Wang Z, Lv Q (2020) miR-101-3p induces vascular endothelial cell dysfunction by targeting tet methylcytosine dioxygenase 2. Acta Biochim Biophys Sin (Shanghai) 52(2):180–191. https://doi.org/10.1093/abbs/gmz154
Weksler B, Romero IA, Couraud PO (2013) The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10(1):16. https://doi.org/10.1186/2045-8118-10-16
Noria S, Cowan DB, Gotlieb AI, Langille BL (1999) Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res 85(6):504–514. https://doi.org/10.1161/01.res.85.6.504
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129(1):203–217. https://doi.org/10.1083/jcb.129.1.203
Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA (2004) VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 286(6):L1143–L1153. https://doi.org/10.1152/ajplung.00305.2003
McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD et al (2007) Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem 103(6):2540–2555. https://doi.org/10.1111/j.1471-4159.2007.04943.x
Dobrogowska DH, Vorbrodt AW (2004) Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood-brain barrier. J Mol Histol 35(5):529–539. https://doi.org/10.1007/10.1007/s10735-004-1318-3
Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP (2004) Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res 1027(1–2):48–58. https://doi.org/10.1016/j.brainres.2004.08.043
Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP (2005) Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol 289(2):H738–H743. https://doi.org/10.1152/ajpheart.01288.2004
Cornford EM, Hyman S (2005) Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx 2(1):27–43. https://doi.org/10.1602/neurorx.2.1.27
Acknowledgements
The authors are highly thankful to Dr. Meghana Rastogi and Ms. Neha Pandey for providing their crucial help during the experiments and manuscript drafting. The authors acknowledge the support provided through the Institute of Eminence Scheme (IoE-6031) of Banaras Hindu University, Varanasi, India.
Author information
Authors and Affiliations
Contributions
UB designed and conducted experiments, performed data analysis, and wrote the initial draft of the manuscript. SKS conceived the idea, supervised the experiments, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhardwaj, U., Singh, S.K. Zika Virus NS1 Suppresses VE-Cadherin and Claudin-5 via hsa-miR-101-3p in Human Brain Microvascular Endothelial Cells. Mol Neurobiol 58, 6290–6303 (2021). https://doi.org/10.1007/s12035-021-02548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02548-x